Structural and biochemical characterization of the Cutibacterium acnes exo-β-1,4-mannosidase that targets the N-glycan core of host glycoproteins
- PMID: 30261037
- PMCID: PMC6160142
- DOI: 10.1371/journal.pone.0204703
Structural and biochemical characterization of the Cutibacterium acnes exo-β-1,4-mannosidase that targets the N-glycan core of host glycoproteins
Abstract
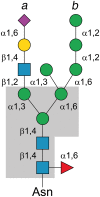
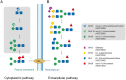
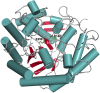
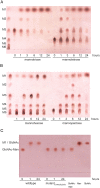
Commensal and pathogenic bacteria have evolved efficient enzymatic pathways to feed on host carbohydrates, including protein-linked glycans. Most proteins of the human innate and adaptive immune system are glycoproteins where the glycan is critical for structural and functional integrity. Besides enabling nutrition, the degradation of host N-glycans serves as a means for bacteria to modulate the host's immune system by for instance removing N-glycans on immunoglobulin G. The commensal bacterium Cutibacterium acnes is a gram-positive natural bacterial species of the human skin microbiota. Under certain circumstances, C. acnes can cause pathogenic conditions, acne vulgaris, which typically affects 80% of adolescents, and can become critical for immunosuppressed transplant patients. Others have shown that C. acnes can degrade certain host O-glycans, however, no degradation pathway for host N-glycans has been proposed. To investigate this, we scanned the C. acnes genome and were able to identify a set of gene candidates consistent with a cytoplasmic N-glycan-degradation pathway of the canonical eukaryotic N-glycan core. We also found additional gene sequences containing secretion signals that are possible candidates for initial trimming on the extracellular side. Furthermore, one of the identified gene products of the cytoplasmic pathway, AEE72695, was produced and characterized, and found to be a functional, dimeric exo-β-1,4-mannosidase with activity on the β-1,4 glycosidic bond between the second N-acetylglucosamine and the first mannose residue in the canonical eukaryotic N-glycan core. These findings corroborate our model of the cytoplasmic part of a C. acnes N-glycan degradation pathway.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Enterococcus faecalis α1-2-mannosidase (EfMan-I): an efficient catalyst for glycoprotein N-glycan modification.FEBS Lett. 2020 Feb;594(3):439-451. doi: 10.1002/1873-3468.13618. Epub 2019 Oct 8. FEBS Lett. 2020. PMID: 31552675 Free PMC article.
-
Crystal structure of a class I alpha1,2-mannosidase involved in N-glycan processing and endoplasmic reticulum quality control.EMBO J. 2000 Feb 15;19(4):581-8. doi: 10.1093/emboj/19.4.581. EMBO J. 2000. PMID: 10675327 Free PMC article.
-
ER-resident protein 46 (ERp46) triggers the mannose-trimming activity of ER degradation-enhancing α-mannosidase-like protein 3 (EDEM3).J Biol Chem. 2018 Jul 6;293(27):10663-10674. doi: 10.1074/jbc.RA118.003129. Epub 2018 May 21. J Biol Chem. 2018. PMID: 29784879 Free PMC article.
-
Staphylococcus epidermidis: A Potential New Player in the Physiopathology of Acne?Dermatology. 2019;235(4):287-294. doi: 10.1159/000499858. Epub 2019 May 21. Dermatology. 2019. PMID: 31112983 Review.
-
Human protein paucimannosylation: cues from the eukaryotic kingdoms.Biol Rev Camb Philos Soc. 2019 Dec;94(6):2068-2100. doi: 10.1111/brv.12548. Epub 2019 Aug 14. Biol Rev Camb Philos Soc. 2019. PMID: 31410980 Review.
Cited by
-
Metagenomic insights into the response of soil microbial communities to pathogenic Ralstonia solanacearum.Front Plant Sci. 2024 Feb 16;15:1325141. doi: 10.3389/fpls.2024.1325141. eCollection 2024. Front Plant Sci. 2024. PMID: 38434434 Free PMC article.
-
Crystal structure of a homotrimeric verrucomicrobial exo-β-1,4-mannosidase active in the hindgut of the wood-feeding termite Reticulitermes flavipes.J Struct Biol X. 2021 May 24;5:100048. doi: 10.1016/j.yjsbx.2021.100048. eCollection 2021. J Struct Biol X. 2021. PMID: 34195602 Free PMC article.
-
Phyllosphere bacterial community dynamics in response to bacterial wildfire disease: succession and interaction patterns.Front Plant Sci. 2024 Mar 12;15:1331443. doi: 10.3389/fpls.2024.1331443. eCollection 2024. Front Plant Sci. 2024. PMID: 38533399 Free PMC article.
-
Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors.Microorganisms. 2021 Feb 2;9(2):303. doi: 10.3390/microorganisms9020303. Microorganisms. 2021. PMID: 33540667 Free PMC article. Review.
-
Mechanism of high-mannose N-glycan breakdown and metabolism by Bifidobacterium longum.Nat Chem Biol. 2023 Feb;19(2):218-229. doi: 10.1038/s41589-022-01202-4. Epub 2022 Nov 28. Nat Chem Biol. 2023. PMID: 36443572 Free PMC article.
References
-
- Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473: 4–8. - PubMed
-
- Walsh CT, Garneau-Tsodikova S, Gatto GJ Jr. Protein posttranslational modifications: The chemistry of proteome diversifications. Angew Chem Int Ed. 2005;44: 7342–7372. - PubMed
-
- Jung D, Alt FW. Unraveling V(D)J recombination: Insights into gene regulation. Cell. 2004;116: 299–311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

