Phospholamban regulates nuclear Ca2+ stores and inositol 1,4,5-trisphosphate mediated nuclear Ca2+ cycling in cardiomyocytes
- PMID: 30261161
- PMCID: PMC6205300
- DOI: 10.1016/j.yjmcc.2018.09.008
Phospholamban regulates nuclear Ca2+ stores and inositol 1,4,5-trisphosphate mediated nuclear Ca2+ cycling in cardiomyocytes
Abstract
Aims: Phospholamban (PLB) is the key regulator of the cardiac Ca2+ pump (SERCA2a)-mediated sarcoplasmic reticulum Ca2+ stores. We recently reported that PLB is highly concentrated in the nuclear envelope (NE) from where it can modulate perinuclear Ca2+ handling of the cardiomyocytes (CMs). Since inositol 1,4,5-trisphosphate (IP3) receptor (IP3R) mediates nuclear Ca2+ release, we examined whether the nuclear pool of PLB regulates IP3-induced nuclear Ca2+ handling.
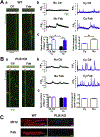
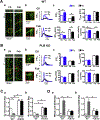
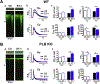
Methods and results: Fluo-4 based confocal Ca2+ imaging was performed to measure Ca2+ dynamics across both nucleus and cytosol in saponin-permeabilized CMs isolated from wild-type (WT) or PLB-knockout (PLB-KO) mice. At diastolic intracellular Ca2+ ([Ca2+]i = 100 nM), the Fab fragment of the monoclonal PLB antibody (anti-PLB Fab) facilitated the formation and increased the length of spontaneous Ca2+ waves (SCWs) originating from the nuclear region in CMs from WT but not from PLB-KO mice. We next examined nuclear Ca2+ activities at basal condition and after sequential addition of IP3, anti-PLB Fab, and the IP3R inhibitor 2-aminoethoxydiphenyl borate (2-APB) at a series of [Ca2+]i. In WT mice, at 10 nM [Ca2+]i where ryanodine receptor (RyR2) based spontaneous Ca2+ sparks rarely occurred, IP3 increased fluorescence amplitude (F/F0) of overall nuclear region to 1.19 ± 0.02. Subsequent addition of anti-PLB Fab significantly decreased F/F0 to 1.09 ± 0.02. At 50 nM [Ca2+]i, anti-PLB Fab not only decreased the overall nuclear F/F0 previously elevated by IP3, but also increased the amplitude and duration of spark-like nuclear Ca2+ release events. These nuclear Ca2+ releases were blocked by 2-APB. At 100 nM [Ca2+]i, IP3 induced short SCWs originating from nucleus. Anti-PLB Fab transformed those short waves into long SCWs with propagation from the nucleus into the cytosol. In contrast, neither nuclear nor cytosolic Ca2+ dynamics was affected by anti-PLB Fab in CMs from PLB-KO mice in all these conditions. Furthermore, in WT CMs pretreated with RyR2 blocker tetracaine, IP3 and anti-PLB Fab still increased the magnitude of nuclear Ca2+ release but failed to regenerate SCWs. Finally, anti-PLB Fab increased low Ca2+ affinity mag-fluo 4 fluorescence intensity in the lumen of NE of nuclei isolated from WT but not in PLB-KO mice.
Conclusion: PLB regulates nuclear Ca2+ handling. By increasing Ca2+ uptake into lumen of the NE and perhaps other perinuclear membranes, the acute reversal of PLB inhibition decreases global Ca2+ concentration at rest in the nucleoplasm, and increases Ca2+ release into the nucleus, through mechanisms involving IP3R and RyR2 in the vicinity.
Keywords: 1,4,5-Trisphosphate receptor; Calcium signaling; Cardiomyocyte; Nuclear Ca(2+) dynamics; Nuclear membranes; Phospholamban; Sarcoplasmic reticulum Ca(2+) cycling.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures

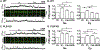



References
-
- Simmerman HK, Jones LR, Phospholamban: protein structure, mechanism of action, and role in cardiac function, Physiol. Rev 78(4) (1998) 921–47. - PubMed
-
- MacLennan DH, Kranias EG, Phospholamban: a crucial regulator of cardiac contractility, Nat. Rev. Mol. Cell. Biol 4(7) (2003) 566–77. - PubMed
-
- Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG, Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation, Circ Res 75(3) (1994) 401–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

