Liposomal Formulations to Modulate the Tumour Microenvironment and Antitumour Immune Response
- PMID: 30261606
- PMCID: PMC6213379
- DOI: 10.3390/ijms19102922
Liposomal Formulations to Modulate the Tumour Microenvironment and Antitumour Immune Response
Abstract
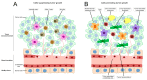
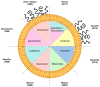
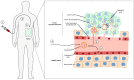
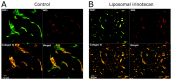
Tumours are complex systems of genetically diverse malignant cells that proliferate in the presence of a heterogeneous microenvironment consisting of host derived microvasculature, stromal, and immune cells. The components of the tumour microenvironment (TME) communicate with each other and with cancer cells, to regulate cellular processes that can inhibit, as well as enhance, tumour growth. Therapeutic strategies have been developed to modulate the TME and cancer-associated immune response. However, modulating compounds are often insoluble (aqueous solubility of less than 1 mg/mL) and have suboptimal pharmacokinetics that prevent therapeutically relevant drug concentrations from reaching the appropriate sites within the tumour. Nanomedicines and, in particular, liposomal formulations of relevant drug candidates, define clinically meaningful drug delivery systems that have the potential to ensure that the right drug candidate is delivered to the right area within tumours at the right time. Following encapsulation in liposomes, drug candidates often display extended plasma half-lives, higher plasma concentrations and may accumulate directly in the tumour tissue. Liposomes can normalise the tumour blood vessel structure and enhance the immunogenicity of tumour cell death; relatively unrecognised impacts associated with using liposomal formulations. This review describes liposomal formulations that affect components of the TME. A focus is placed on formulations which are approved for use in the clinic. The concept of tumour immunogenicity, and how liposomes may enhance radiation and chemotherapy-induced immunogenic cell death (ICD), is discussed. Liposomes are currently an indispensable tool in the treatment of cancer, and their contribution to cancer therapy may gain even further importance by incorporating modulators of the TME and the cancer-associated immune response.
Keywords: doxorubicin; immunogenic cell death; irinotecan; liposomes; mifamurtide; paclitaxel; radiotherapy; tumour microenvironment; tumour stroma; tumour vasculature; tumour-infiltrating lymphocytes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Role of liposomes in chemoimmunotherapy of breast cancer.J Drug Target. 2025 Jul;33(6):887-915. doi: 10.1080/1061186X.2025.2467139. Epub 2025 Mar 3. J Drug Target. 2025. PMID: 39967479 Review.
-
Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours.Drugs. 1997;54 Suppl 4:15-21. doi: 10.2165/00003495-199700544-00005. Drugs. 1997. PMID: 9361957 Review.
-
The liposomal formulation of doxorubicin.Methods Enzymol. 2005;391:71-97. doi: 10.1016/S0076-6879(05)91004-5. Methods Enzymol. 2005. PMID: 15721375
-
Liposomes targeting tumour stromal cells.Mol Membr Biol. 2010 Oct;27(7):328-40. doi: 10.3109/09687688.2010.522204. Epub 2010 Oct 13. Mol Membr Biol. 2010. PMID: 20939769 Review.
-
Modifying the tumour microenvironment and reverting tumour cells: New strategies for treating malignant tumours.Cell Prolif. 2020 Aug;53(8):e12865. doi: 10.1111/cpr.12865. Epub 2020 Jun 26. Cell Prolif. 2020. PMID: 32588948 Free PMC article. Review.
Cited by
-
Nanomaterials-Mediated Immunomodulation for Cancer Therapeutics.Front Chem. 2021 Feb 23;9:629635. doi: 10.3389/fchem.2021.629635. eCollection 2021. Front Chem. 2021. PMID: 33708759 Free PMC article. Review.
-
Interventional nanotheranostics in hepatocellular carcinoma.Nanotheranostics. 2023 Jan 9;7(2):128-141. doi: 10.7150/ntno.80120. eCollection 2023. Nanotheranostics. 2023. PMID: 36793354 Free PMC article. Review.
-
Do Lipid-based Nanoparticles Hold Promise for Advancing the Clinical Translation of Anticancer Alkaloids?Cancers (Basel). 2021 Oct 25;13(21):5346. doi: 10.3390/cancers13215346. Cancers (Basel). 2021. PMID: 34771511 Free PMC article. Review.
-
The yin and yang functions of extracellular ATP and adenosine in tumor immunity.Cancer Cell Int. 2020 Apr 7;20:110. doi: 10.1186/s12935-020-01195-x. eCollection 2020. Cancer Cell Int. 2020. PMID: 32280302 Free PMC article. Review.
-
Advances in Materials Science for Precision Melanoma Therapy: Nanotechnology-Enhanced Drug Delivery Systems.Pharmaceutics. 2025 Feb 24;17(3):296. doi: 10.3390/pharmaceutics17030296. Pharmaceutics. 2025. PMID: 40142960 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

