Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined
- PMID: 30261630
- PMCID: PMC6215107
- DOI: 10.3390/toxins10100392
Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined
Abstract
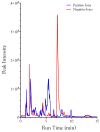
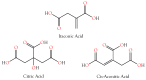
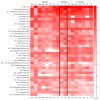
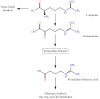
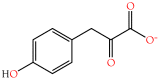
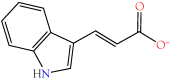
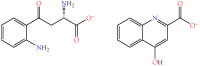
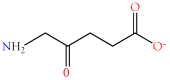
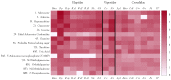
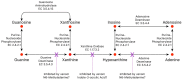
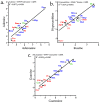
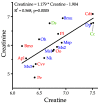
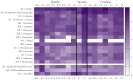
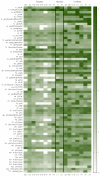
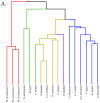
Small metabolites and peptides in 17 snake venoms (Elapidae, Viperinae, and Crotalinae), were quantified using liquid chromatography-mass spectrometry. Each venom contains >900 metabolites and peptides. Many small organic compounds are present at levels that are probably significant in prey envenomation, given that their known pharmacologies are consistent with snake envenomation strategies. Metabolites included purine nucleosides and their bases, neurotransmitters, neuromodulators, guanidino compounds, carboxylic acids, amines, mono- and disaccharides, and amino acids. Peptides of 2⁻15 amino acids are also present in significant quantities, particularly in crotaline and viperine venoms. Some constituents are specific to individual taxa, while others are broadly distributed. Some of the latter appear to support high anabolic activity in the gland, rather than having toxic functions. Overall, the most abundant organic metabolite was citric acid, owing to its predominance in viperine and crotaline venoms, where it chelates divalent cations to prevent venom degradation by venom metalloproteases and damage to glandular tissue by phospholipases. However, in terms of their concentrations in individual venoms, adenosine, adenine, were most abundant, owing to their high titers in Dendroaspis polylepis venom, although hypoxanthine, guanosine, inosine, and guanine all numbered among the 50 most abundant organic constituents. A purine not previously reported in venoms, ethyl adenosine carboxylate, was discovered in D. polylepis venom, where it probably contributes to the profound hypotension caused by this venom. Acetylcholine was present in significant quantities only in this highly excitotoxic venom, while 4-guanidinobutyric acid and 5-guanidino-2-oxopentanoic acid were present in all venoms.
Keywords: amines; amino acids; carboxylic acids; guanidinium compounds; metabolites; mono- and disaccharides; neuromodulators; neurotransmitters; peptides; purine nucleosides and bases; snake venoms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Taxonomic distribution and quantitative analysis of free purine and pyrimidine nucleosides in snake venoms.Comp Biochem Physiol B Biochem Mol Biol. 2005 Jan;140(1):109-26. doi: 10.1016/j.cbpc.2004.09.020. Comp Biochem Physiol B Biochem Mol Biol. 2005. PMID: 15621516 Review.
-
Ophidian envenomation strategies and the role of purines.Toxicon. 2002 Apr;40(4):335-93. doi: 10.1016/s0041-0101(01)00232-x. Toxicon. 2002. PMID: 11738231 Review.
-
Venomous snakes of Costa Rica: biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics.J Proteomics. 2014 Jun 13;105:323-39. doi: 10.1016/j.jprot.2014.02.020. Epub 2014 Feb 24. J Proteomics. 2014. PMID: 24576642 Review.
-
Venom peptide analysis of Vipera ammodytes meridionalis (Viperinae) and Bothrops jararacussu (Crotalinae) demonstrates subfamily-specificity of the peptidome in the family Viperidae.Mol Biosyst. 2011 Dec;7(12):3298-307. doi: 10.1039/c1mb05309d. Epub 2011 Sep 29. Mol Biosyst. 2011. PMID: 21959992
-
Proteomic characterization of six Taiwanese snake venoms: Identification of species-specific proteins and development of a SISCAPA-MRM assay for cobra venom factors.J Proteomics. 2018 Sep 15;187:59-68. doi: 10.1016/j.jprot.2018.06.003. Epub 2018 Jun 19. J Proteomics. 2018. PMID: 29929037
Cited by
-
Antimicrobial peptidomes of Bothrops atrox and Bothrops jararacussu snake venoms.Amino Acids. 2021 Oct;53(10):1635-1648. doi: 10.1007/s00726-021-03055-y. Epub 2021 Sep 4. Amino Acids. 2021. PMID: 34482475
-
Metabolomics and proteomics: synergistic tools for understanding snake venom inhibition.Arch Toxicol. 2025 Mar;99(3):915-934. doi: 10.1007/s00204-024-03947-4. Epub 2025 Jan 6. Arch Toxicol. 2025. PMID: 39760869 Review.
-
Mass-Spectrometry-Based Lipidome and Proteome Profiling of Hottentotta saulcyi (Scorpiones: Buthidae) Venom.Toxins (Basel). 2022 May 26;14(6):370. doi: 10.3390/toxins14060370. Toxins (Basel). 2022. PMID: 35737031 Free PMC article.
-
Extracellular Vesicles from Bothrops jararaca Venom Are Diverse in Structure and Protein Composition and Interact with Mammalian Cells.Toxins (Basel). 2022 Nov 19;14(11):806. doi: 10.3390/toxins14110806. Toxins (Basel). 2022. PMID: 36422980 Free PMC article.
-
Identification and quantification of honeybee venom constituents by multiplatform metabolomics.Sci Rep. 2020 Dec 10;10(1):21645. doi: 10.1038/s41598-020-78740-1. Sci Rep. 2020. PMID: 33303913 Free PMC article.
References
-
- Ganguly S.N., Malkana M.T. Indian snake venoms. II. Cobra venom: Its chemical composition, protein fractions and their physiological actions. Indian J. Med. Res. 1936;24:281–286.
-
- Devi A. The protein and nonprotein constituents of snake venoms. In: Bücherl W., Buckley E.E., Deulofeu V., editors. Venomous Animals and Their Venoms. Volume 1. Academic Press; New York, NY, USA: 1968. pp. 119–165.
-
- Bieber A.L. Metal and Nonprotein Constituents in Snake Venoms. In: Md C.-Y.L., editor. Snake Venoms. Handbook of Experimental Pharmacology; Springer; Berlin/Heidelberg, Germany: 1979. pp. 295–306.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

