Cis-3-O-p- hydroxycinnamoyl Ursolic Acid Induced ROS-Dependent p53-Mediated Mitochondrial Apoptosis in Oral Cancer Cells
- PMID: 30261716
- PMCID: PMC6319548
- DOI: 10.4062/biomolther.2017.237
Cis-3-O-p- hydroxycinnamoyl Ursolic Acid Induced ROS-Dependent p53-Mediated Mitochondrial Apoptosis in Oral Cancer Cells
Abstract
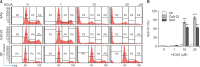
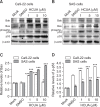
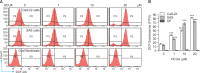
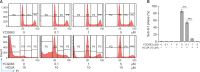
Cis-3-O-p-hydroxycinnamoyl ursolic acid (HCUA), a triterpenoid compound, was purified from Elaeagnus oldhamii Maxim. This traditional medicinal plant has been used for treating rheumatoid arthritis and lung disorders as well as for its anti-inflammation and anticancer activities. This study aimed to investigate the anti-proliferative and apoptotic-inducing activities of HCUA in oral cancer cells. HCUA exhibited anti-proliferative activity in oral cancer cell lines (Ca9-22 and SAS cells), but not in normal oral fibroblasts. The inhibitory concentration of HCUA that resulted in 50% viability was 24.0 µM and 17.8 µM for Ca9-22 and SAS cells, respectively. Moreover, HCUA increased the number of cells in the sub-G1 arrest phase and apoptosis in a concentration-dependent manner in both oral cancer cell lines, but not in normal oral fibroblasts. Importantly, HCUA induced p53-mediated transcriptional regulation of pro-apoptotic proteins (Bax, Bak, Bim, Noxa, and PUMA), which are associated with mitochondrial apoptosis in oral cancer cells via the loss of mitochondrial membrane potential. HCUA triggered the production of intracellular reactive oxygen species (ROS) that was ascertained to be involved in HCUA-induced apoptosis by the ROS inhibitors YCG063 and N-acetyl-L-cysteine. As a result, HCUA had potential antitumor activity to oral cancer cells through eliciting ROS-dependent and p53-mediated mitochondrial apoptosis. Overall, HCUA could be applicable for the development of anticancer agents against human oral cancer.
Keywords: Cis-3-O-p-hydroxycinnamoyl ursolic acid; Mitochondrial apoptosis; Oral cancer cells; ROS.
Conflict of interest statement
All authors had no conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

