MRI predictors of amyloid pathology: results from the EMIF-AD Multimodal Biomarker Discovery study
- PMID: 30261928
- PMCID: PMC6161396
- DOI: 10.1186/s13195-018-0428-1
MRI predictors of amyloid pathology: results from the EMIF-AD Multimodal Biomarker Discovery study
Abstract
Background: With the shift of research focus towards the pre-dementia stage of Alzheimer's disease (AD), there is an urgent need for reliable, non-invasive biomarkers to predict amyloid pathology. The aim of this study was to assess whether easily obtainable measures from structural MRI, combined with demographic data, cognitive data and apolipoprotein E (APOE) ε4 genotype, can be used to predict amyloid pathology using machine-learning classification.
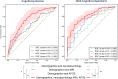
Methods: We examined 810 subjects with structural MRI data and amyloid markers from the European Medical Information Framework for Alzheimer's Disease Multimodal Biomarker Discovery study, including subjects with normal cognition (CN, n = 337, age 66.5 ± 7.2, 50% female, 27% amyloid positive), mild cognitive impairment (MCI, n = 375, age 69.1 ± 7.5, 53% female, 63% amyloid positive) and AD dementia (n = 98, age 67.0 ± 7.7, 48% female, 97% amyloid positive). Structural MRI scans were visually assessed and Freesurfer was used to obtain subcortical volumes, cortical thickness and surface area measures. We first assessed univariate associations between MRI measures and amyloid pathology using mixed models. Next, we developed and tested an automated classifier using demographic, cognitive, MRI and APOE ε4 information to predict amyloid pathology. A support vector machine (SVM) with nested 10-fold cross-validation was applied to identify a set of markers best discriminating between amyloid positive and amyloid negative subjects.
Results: In univariate associations, amyloid pathology was associated with lower subcortical volumes and thinner cortex in AD-signature regions in CN and MCI. The multi-variable SVM classifier provided an area under the curve (AUC) of 0.81 ± 0.07 in MCI and an AUC of 0.74 ± 0.08 in CN. In CN, selected features for the classifier included APOE ε4, age, memory scores and several MRI measures such as hippocampus, amygdala and accumbens volumes and cortical thickness in temporal and parahippocampal regions. In MCI, the classifier including demographic and APOE ε4 information did not improve after additionally adding imaging measures.
Conclusions: Amyloid pathology is associated with changes in structural MRI measures in CN and MCI. An automated classifier based on clinical, imaging and APOE ε4 data can identify the presence of amyloid pathology with a moderate level of accuracy. These results could be used in clinical trials to pre-screen subjects for anti-amyloid therapies.
Keywords: Alzheimer’s disease; Amyloid; Biomarkers; European Medical Information Framework for Alzheimer’s Disease; Machine learning; Magnetic resonance imaging; Mild cognitive impairment; Support vector machine.
Conflict of interest statement
Ethics approval and consent to participate
The local medical ethics committee in each centre approved the study. Subjects had already provided written informed consent at the time of inclusion in the cohort for use of data, samples and scans.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. HZ has served on scientific advisory boards of Eli Lilly and Roche Diagnostics, has received travel support from Teva and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. MFG’s current employer is Teva Pharmaceuticals, Inc., Malvern, PA, USA; his former employer was Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA. Any views expressed in this publication represent the personal opinions of the authors and not those of their respective employer. JCR is a full-time employee of GlaxoSmithkline. PM-L reports personal fees from Lilly, Axon, General Electric and Nutricia for advisory boards, and lecturing fees from Lilly, Nutricia, Piramal. RV was principal investigator of the phase 1 and 2 [18F]flutemetamol trials. RV’s institution has clinical trial agreements (RV as PI) with AbbVie, Biogen, EliLilly, Merck and Novartis, and consultancy agreements (RV as PI) with Novartis and Cytox Ltd. SL has done consultancy for Eaisi, EIP Pharma, SomaLogic, Merck and Optum Labs.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Ritchie CW, Molinuevo JL, Truyen L, Satlin A, Van der Geyten S, Lovestone S, et al. Development of interventions for the secondary prevention of Alzheimer’s dementia: the European Prevention of Alzheimer’s Dementia (EPAD) project. Lancet Psychiatry. 2016;3:179–186. doi: 10.1016/S2215-0366(15)00454-X. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

