THROMBOTECT - a randomized study comparing low molecular weight heparin, antithrombin and unfractionated heparin for thromboprophylaxis during induction therapy of acute lymphoblastic leukemia in children and adolescents
- PMID: 30262570
- PMCID: PMC6442986
- DOI: 10.3324/haematol.2018.194175
THROMBOTECT - a randomized study comparing low molecular weight heparin, antithrombin and unfractionated heparin for thromboprophylaxis during induction therapy of acute lymphoblastic leukemia in children and adolescents
Abstract
Thromboembolism is a serious complication of induction therapy for childhood acute lymphoblastic leukemia. We prospectively compared the efficacy and safety of antithrombotic interventions in the consecutive leukemia trials ALL-BFM 2000 and AIEOP-BFM ALL 2009. Patients with newly diagnosed acute lymphoblastic leukemia (n=949, age 1 to 18 years) were randomized to receive low-dose unfractionated heparin, prophylactic low molecular weight heparin (enoxaparin) or activity-adapted antithrombin throughout induction therapy. The primary objective of the study was to determine whether enoxaparin or antithrombin reduces the incidence of thromboembolism as compared to unfractionated heparin. The principal safety outcome was hemorrhage; leukemia outcome was a secondary endpoint. Thromboembolism occurred in 42 patients (4.4%). Patients assigned to unfractionated heparin had a higher risk of thromboembolism (8.0%) compared with those randomized to enoxaparin (3.5%; P=0.011) or antithrombin (1.9%; P<0.001). The proportion of patients who refused antithrombotic treatment as allocated was 3% in the unfractionated heparin or antithrombin arms, and 33% in the enoxaparin arm. Major hemorrhage occurred in eight patients (no differences between the groups). The 5-year event-free survival was 80.9±2.2% among patients assigned to antithrombin compared to 85.9±2.0% in the unfractionated heparin group (P=0.06), and 86.2±2.0% in the enoxaparin group (P=0.10). In conclusion, prophylactic use of antithrombin or enoxaparin significantly reduced thromboembolism. Despite the considerable number of patients rejecting the assigned treatment with subcutaneous injections, the result remains unambiguous. Thromboprophylaxis - for the present time primarily with enoxaparin - can be recommended for children and adolescents with acute lymphoblastic leukemia during induction therapy. Whether and how antithrombin may affect leukemia outcome remains to be determined.
Copyright© 2019 Ferrata Storti Foundation.
Figures
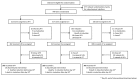
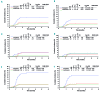

Comment in
-
THROMBOTECT takes the lead.Haematologica. 2019 Apr;104(4):644-645. doi: 10.3324/haematol.2018.209528. Haematologica. 2019. PMID: 30930335 Free PMC article. No abstract available.
References
-
- Athale UH, Chan AK. Thrombosis in children with acute lymphoblastic leukemia. Part I. Epidemiology of thrombosis in children with acute lymphoblastic leukemia. Thromb Res. 2003;111(3):125–131. - PubMed
-
- Mitchell L, Hoogendoorn H, Giles AR, Vegh P, Andrew M. Increased endogenous thrombin generation in children with acute lymphoblastic leukemia: risk of thrombotic complications in L-asparaginase-induced antithrombin III deficiency. Blood. 1994;83(2):386–391. - PubMed
-
- Mitchell LG, Sutor AH, Andrew M. Hemostasis in childhood acute lymphoblastic leukemia: coagulopathy induced by disease and treatment. Semin Thromb Hemost. 1995;21(4):390–401. - PubMed
-
- Nowak-Göttl U, Ahlke E, Fleischhack G, et al. Thromboembolic events in children with acute lymphoblastic leukemia (BFM protocols): prednisone versus dexamethasone administration. Blood. 2003;101(7):2529–2533. - PubMed
-
- Caruso V, Iacoviello L, di Castelnuovo A, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108(7):2216–2222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

