Ketamine, but Not the NMDAR Antagonist Lanicemine, Increases Prefrontal Global Connectivity in Depressed Patients
- PMID: 30263977
- PMCID: PMC6154502
- DOI: 10.1177/2470547018796102
Ketamine, but Not the NMDAR Antagonist Lanicemine, Increases Prefrontal Global Connectivity in Depressed Patients
Abstract
Background: Identifying the neural correlates of ketamine treatment may facilitate and expedite the development of novel, robust, and safe rapid-acting antidepressants. Prefrontal cortex (PFC) global brain connectivity with global signal regression (GBCr) was recently identified as a putative biomarker of major depressive disorder (MDD). Accumulating evidence have repeatedly shown reduced PFC GBCr in MDD, an abnormality which appears to normalize following ketamine treatment.
Methods: Fifty-six unmedicated participants with MDD were randomized to intravenous placebo (normal saline; n = 18), ketamine (0.5mg/kg; n = 19) or lanicemine (100mg; n = 19). PFC GBCr was computed using time series from functional magnetic resonance imaging (fMRI) scans that were completed at baseline, during infusion, and 24h post-treatment.
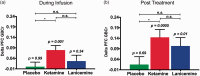
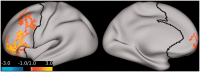
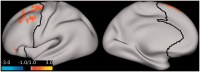
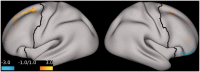
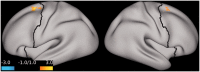
Results: Compared to placebo, ketamine significantly increased average PFC GBCr during infusion (p = 0.01) and 24h post-treatment (p = 0.02). Lanicemine had no significant effects on GBCr during infusion (p = 0.45) and 24h post-treatment (p = 0.23), compared to placebo. Average delta PFC GBCr (during minus baseline) showed a pattern of positively predicting depression improvement in participants receiving ketamine (r = 0.44; p = 0.06; d = 1.0) or lanicemine (r = 0.55; p = 0.01; d = 1.3), but not those receiving placebo (r = -0.1; p = 0.69; d = 0.02). Follow-up vertex-wise analyses showed ketamine-induced GBCr increases in the dorsolateral, dorsomedial, and frontomedial PFC during infusion, and in the dorsolateral and dorsomedial PFC 24h post-treatment (corrected p < 0.05). Exploratory vertex-wise analyses examining the relationship with depression improvement showed positive correlation with GBCr in the dorsal PFC during infusion and 24h post-treatment, but negative correlation with GBCr in the ventral PFC during infusion (uncorrected p < 0.01).
Conclusions: In a randomized placebo-controlled approach, the results provide the first evidence in MDD of ketamine-induced increases in PFC GBCr during infusion, and suggests that ketamine's rapid-acting antidepressant properties are related to its acute effects on prefrontal connectivity. Overall, the study findings underscore the similarity and differences between ketamine and another N-methyl-D-aspartate receptor (NMDAR) antagonist, while proposing a pharmacoimaging paradigm for optimization of novel rapid-acting antidepressants prior to testing in costly clinical trials.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.G.A. has served as a consultant and/or on advisory boards for Genentech and Janssen and as an editor for
Figures
References
-
- Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov 2017; 16: 472–486. - PubMed
-
- Zarate CA, Jr, Singh JB, Quiroz JA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry 2006; 163: 153–155. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

