Prevalence of Variant Reclassification Following Hereditary Cancer Genetic Testing
- PMID: 30264118
- PMCID: PMC6233618
- DOI: 10.1001/jama.2018.13152
Prevalence of Variant Reclassification Following Hereditary Cancer Genetic Testing
Abstract
Importance: Variant reclassification is an important component of hereditary cancer genetic testing; however, there are few published data quantifying the prevalence of reclassification.
Objective: Retrospective cohort study of individuals who had genetic testing from 2006 through 2016 at a single commercial laboratory.
Design, setting, and participants: A retrospective cohort of individuals who had genetic testing between 2006 and 2016 at a single commercial laboratory was assessed. Variants were classified as benign, likely benign, variant of uncertain significance, likely pathogenic, or pathogenic. Retrospective chart reviews were conducted for patients from the University of Texas Southwestern (UTSW) Medical Center.
Exposures: Hereditary cancer genetic testing.
Main outcomes and measures: Frequency of and time to amended reports; frequency and types of variant reclassification.
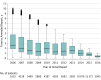
Results: From 2006 through 2018, 1.45 million individuals (median [interquartile range] age at testing, 49 years [40.69-58.31 years], 95.6% women) had genetic testing, and 56.6% (n = 821 724) had a personal history of cancer. A total of 1.67 million initial tests were reported and 59 955 amended reports were issued due to variant reclassification. Overall, 6.4% (2868 of 44 777) of unique variants were reclassified. Reclassification to a different clinical category was rare among unique variants initially classified as pathogenic or likely pathogenic (0.7%, 61 of 9112) or benign or likely benign (0.2%, 15 of 8995). However, 7.7% (2048 of 26 670) of unique variants of uncertain significance were reclassified: 91.2% (1867 of 2048) were downgraded to benign or likely benign (median time to amended report, 1.17 years), 8.7% (178 of 2048) were upgraded to pathogenic or likely pathogenic variants (median time to amended report, 1.86 years). Because most variants were observed in more than 1 individual, 24.9% (46 890 of 184 327) of all reported variants of uncertain significance were reclassified.
Conclusions and relevance: Following hereditary cancer genetic testing at a single commercial laboratory, 24.9% of variants of uncertain significance were reclassified, which included both downgrades and upgrades. Further research is needed to assess generalizability of the findings for other laboratories, as well as the clinical consequences of the reclassification as a component of a genetic testing program.
Conflict of interest statement
Figures
Comment in
-
Making Sense of the Genome Remains a Work in Progress.JAMA. 2018 Sep 25;320(12):1247-1248. doi: 10.1001/jama.2018.11784. JAMA. 2018. PMID: 30264098 No abstract available.
Similar articles
-
Impact of Variant Reclassification in Cancer Predisposition Genes on Clinical Care.JCO Precis Oncol. 2021 Nov;5:577-584. doi: 10.1200/PO.20.00399. JCO Precis Oncol. 2021. PMID: 34994607
-
Rates and Classification of Variants of Uncertain Significance in Hereditary Disease Genetic Testing.JAMA Netw Open. 2023 Oct 2;6(10):e2339571. doi: 10.1001/jamanetworkopen.2023.39571. JAMA Netw Open. 2023. PMID: 37878314 Free PMC article.
-
Assessment of Diagnostic Outcomes of RNA Genetic Testing for Hereditary Cancer.JAMA Netw Open. 2019 Oct 2;2(10):e1913900. doi: 10.1001/jamanetworkopen.2019.13900. JAMA Netw Open. 2019. PMID: 31642931 Free PMC article.
-
Classification and Clinical Management of Variants of Uncertain Significance in High Penetrance Cancer Predisposition Genes.Hum Mutat. 2016 Apr;37(4):331-6. doi: 10.1002/humu.22956. Epub 2016 Feb 5. Hum Mutat. 2016. PMID: 26777316 Review.
-
Sequence Variants of Uncertain Significance: What to Do When Genetic Test Results Are Not Definitive.Surg Oncol Clin N Am. 2015 Oct;24(4):833-46. doi: 10.1016/j.soc.2015.06.009. Surg Oncol Clin N Am. 2015. PMID: 26363543 Review.
Cited by
-
Recontacting registry participants with genetic updates through GenomeConnect, the ClinGen patient registry.Genet Med. 2021 Sep;23(9):1738-1745. doi: 10.1038/s41436-021-01197-8. Epub 2021 May 18. Genet Med. 2021. PMID: 34007001 Free PMC article.
-
BRCA-Mutated Pancreatic Cancer: From Discovery to Novel Treatment Paradigms.Cancers (Basel). 2022 May 16;14(10):2453. doi: 10.3390/cancers14102453. Cancers (Basel). 2022. PMID: 35626055 Free PMC article. Review.
-
Is there a duty to reinterpret genetic data? The ethical dimensions.Genet Med. 2020 Mar;22(3):633-639. doi: 10.1038/s41436-019-0679-7. Epub 2019 Oct 15. Genet Med. 2020. PMID: 31616070 Free PMC article.
-
Landscape of germline pathogenic variants in patients with dual primary breast and lung cancer.Hum Genomics. 2023 Jul 17;17(1):66. doi: 10.1186/s40246-023-00510-7. Hum Genomics. 2023. PMID: 37461096 Free PMC article.
-
Variant Interpretation for Dilated Cardiomyopathy: Refinement of the American College of Medical Genetics and Genomics/ClinGen Guidelines for the DCM Precision Medicine Study.Circ Genom Precis Med. 2020 Apr;13(2):e002480. doi: 10.1161/CIRCGEN.119.002480. Epub 2020 Mar 11. Circ Genom Precis Med. 2020. PMID: 32160020 Free PMC article.
References
-
- Daly M, Pilarski R, Berry M, et al. Genetic/familial high-risk assessment: breast and ovarian. NCCN Clinical Practice Guidelines in Oncology. (Version 2.2017). http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf 2017. Accessed June 1, 2018.
-
- Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi:10.1038/gim.2015.30 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

