Timing of Loading Dose of Atorvastatin in Patients Undergoing Percutaneous Coronary Intervention for Acute Coronary Syndromes: Insights From the SECURE-PCI Randomized Clinical Trial
- PMID: 30264159
- PMCID: PMC6583055
- DOI: 10.1001/jamacardio.2018.3408
Timing of Loading Dose of Atorvastatin in Patients Undergoing Percutaneous Coronary Intervention for Acute Coronary Syndromes: Insights From the SECURE-PCI Randomized Clinical Trial
Erratum in
-
Errors in Abstract, Figure 1, Table, Conclusion, and Author Contributions.JAMA Cardiol. 2018 Nov 1;3(11):1131. doi: 10.1001/jamacardio.2018.3778. JAMA Cardiol. 2018. PMID: 30476979 Free PMC article. No abstract available.
Abstract
Importance: Loading doses of atorvastatin did not show reduction on clinical outcomes in the overall population of patients with acute coronary syndrome (ACS) enrolled in the Statins Evaluation in Coronary Procedures and Revascularization (SECURE-PCI) trial, but a potential benefit was identified in patients who subsequently underwent percutaneous coronary intervention (PCI).
Objectives: To determine whether periprocedural loading doses of atorvastatin are associated with decreased 30-day major adverse cardiovascular events (MACE) in patients with ACS undergoing PCI according to type of ACS and timing of atorvastatin administration before PCI.
Design, setting, and participants: Secondary analysis of a multicenter, double-blind, placebo-controlled, randomized clinical trial conducted at 53 sites that enrolled 4191 patients with ACS intended to be treated with PCI between April 18, 2012, and October 06, 2017.
Interventions: Patients were randomized to 2 loading doses of 80 mg of atorvastatin or matching placebo before and 24 hours after a planned PCI. By protocol, all patients (regardless of treatment group) received 40 mg of atorvastatin for 30 days starting 24 hours after the second dose of study medication.
Main outcomes and measures: The primary outcome was MACE through 30 days, composed by all-cause mortality, myocardial infarction, stroke, and unplanned coronary revascularization. Cox regression models adjusting for key baseline characteristics were used to assess the association between atorvastatin and MACE in patients undergoing PCI.
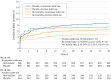
Results: From the overall trial population, 2710 (64.7%) underwent PCI (650 women [24.0%]; mean [SD] age, 62 [11.3] years). Loading atorvastatin was associated with reduced MACE at 30 days by 28% in the PCI group (adjusted hazard ratio [HR], 0.72; 95% CI 0.54-0.97; P = .03). Loading dose of atorvastatin was administered less than 12 hours before PCI in 2548 patients (95.3%) (45.1% < 2 hours and 54.3% between 2 and 12 hours). There was no significant interaction between treatment effect and timing of study drug administration. The treatment effect of loading atorvastatin was more pronounced in patients with ST-segment elevation myocardial infarction than in patients with non-ST-segment elevation ACS (adjusted HR, 0.59; 95% CI, 0.38-0.92; P = .02; HR, 0.85; 95% CI, 0.58-1.27; P = .43, respectively).
Conclusions and relevance: In patients with ACS undergoing PCI, periprocedural loading doses of atorvastatin appeared to reduce the rate of MACE at 30 days, most clearly in patients with ST-segment elevation myocardial infarction. This beneficial effect seemed to be preserved and consistent, irrespective of the timing of atorvastatin administration, including within 2 hours before PCI.
Trial registration: clinicaltrials.gov Identifier: NCT01448642.
Conflict of interest statement
Figures

References
-
- Stone GW, Mehran R, Dangas G, Lansky AJ, Kornowski R, Leon MB. Differential impact on survival of electrocardiographic Q-wave versus enzymatic myocardial infarction after percutaneous intervention: a device-specific analysis of 7147 patients. Circulation. 2001;104(6):642-647. doi: 10.1161/hc3101.093902 - DOI - PubMed
-
- Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G; ARMYDA Investigators . Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2004;110(6):674-678. doi: 10.1161/01.CIR.0000137828.06205.87 - DOI - PubMed
-
- Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49(12):1272-1278. doi: 10.1016/j.jacc.2007.02.025 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

