Activity of two hyaluronan preparations on primary human oral fibroblasts
- PMID: 30264516
- PMCID: PMC6586051
- DOI: 10.1111/jre.12602
Activity of two hyaluronan preparations on primary human oral fibroblasts
Abstract
Background and objective: The potential benefit of using hyaluronan (HA) in reconstructive periodontal surgery is still a matter of debate. The aim of the present study was to evaluate the effects of two HA formulations on human oral fibroblasts involved in soft tissue wound healing/regeneration.
Material and methods: Metabolic, proliferative and migratory abilities of primary human palatal and gingival fibroblasts were examined upon HA treatment. To uncover the mechanisms whereby HA influences cellular behavior, wound healing-related gene expression and activation of signaling kinases were analyzed by qRT-PCR and immunoblotting, respectively.
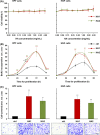
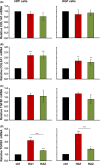
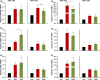
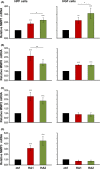
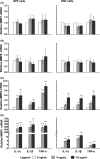
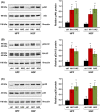
Results: The investigated HA formulations maintained the viability of oral fibroblasts and increased their proliferative and migratory abilities. They enhanced expression of genes encoding type III collagen and transforming growth factor-β3, characteristic of scarless wound healing. The HAs upregulated the expression of genes encoding pro-proliferative, pro-migratory, and pro-inflammatory factors, with only a moderate effect on the latter in gingival fibroblasts. In palatal but not gingival fibroblasts, an indirect effect of HA on the expression of matrix metalloproteinases 2 and 3 was detected, potentially exerted through induction of pro-inflammatory cytokines. Finally, our data pointed on Akt, Erk1/2 and p38 as the signaling molecules whereby the HAs exert their effects on oral fibroblasts.
Conclusion: Both investigated HA formulations are biocompatible and enhance the proliferative, migratory and wound healing properties of cell types involved in soft tissue wound healing following regenerative periodontal surgery. Our data further suggest that in gingival tissues, the HAs are not likely to impair the healing process by prolonging inflammation or causing excessive MMP expression at the repair site.
Keywords: gene expression; growth factors; hyaluronic acid; oral soft tissue wound healing; pro-inflammatory cytokines.
© 2018 The Authors. Journal of Periodontal Research Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no conflicts of interest related to this study.
Figures
Similar articles
-
Differential effects of hyaluronan and its fragments on fibroblasts: relation to wound healing.Wound Repair Regen. 2008 Mar-Apr;16(2):274-87. doi: 10.1111/j.1524-475X.2007.00342.x. Epub 2008 Feb 13. Wound Repair Regen. 2008. PMID: 18282267
-
Morphological and Molecular Evaluation of a Gel Based on Hyaluronic Acid and Spermidine for Oral Regenerative Purposes.Cells. 2025 Jul 9;14(14):1047. doi: 10.3390/cells14141047. Cells. 2025. PMID: 40710300 Free PMC article.
-
High molecular weight hyaluronic acid regulates P. gingivalis-induced inflammation and migration in human gingival fibroblasts via MAPK and NF-κB signaling pathway.Arch Oral Biol. 2019 Feb;98:75-80. doi: 10.1016/j.archoralbio.2018.10.027. Epub 2018 Nov 1. Arch Oral Biol. 2019. PMID: 30465936
-
Subpopulations of fetal-like gingival fibroblasts: characterisation and potential significance for wound healing and the progression of periodontal disease.Oral Dis. 1996 Jun;2(2):155-66. doi: 10.1111/j.1601-0825.1996.tb00217.x. Oral Dis. 1996. PMID: 8957929 Review.
-
Multiple functions of gingival and mucoperiosteal fibroblasts in oral wound healing and repair.Periodontol 2000. 2015 Jun;68(1):21-40. doi: 10.1111/prd.12076. Periodontol 2000. 2015. PMID: 25867977 Review.
Cited by
-
Hyaluronic Acid as an Adjunct to Coronally Advanced Flap Procedures for Gingival Recessions: A Systematic Review and Meta-Analysis of Randomized Clinical Trials.J Pers Med. 2022 Sep 19;12(9):1539. doi: 10.3390/jpm12091539. J Pers Med. 2022. PMID: 36143324 Free PMC article. Review.
-
Treatment of Intrabony Defects with a Combination of Hyaluronic Acid and Deproteinized Porcine Bone Mineral.Materials (Basel). 2021 Nov 11;14(22):6795. doi: 10.3390/ma14226795. Materials (Basel). 2021. PMID: 34832196 Free PMC article.
-
Biological molecules in dental applications: hyaluronic acid as a companion biomaterial for diverse dental applications.Heliyon. 2020 Apr 6;6(4):e03722. doi: 10.1016/j.heliyon.2020.e03722. eCollection 2020 Apr. Heliyon. 2020. PMID: 32280803 Free PMC article. Review.
-
Hyaluronic acid in Dentoalveolar regeneration: Biological rationale and clinical applications.J Oral Biol Craniofac Res. 2024 Mar-Apr;14(2):230-235. doi: 10.1016/j.jobcr.2024.02.010. Epub 2024 Mar 12. J Oral Biol Craniofac Res. 2024. PMID: 38510340 Free PMC article.
-
Microbiological Effects of Sodium Hypochlorite/-Amino Acids and Cross-linked Hyaluronic Acid Adjunctive to Non-surgical Periodontal Treatment.Oral Health Prev Dent. 2024 Apr 30;22:171-180. doi: 10.3290/j.ohpd.b5281925. Oral Health Prev Dent. 2024. PMID: 38687029 Free PMC article. Clinical Trial.
References
-
- Embery G, Oliver WM, Stanbury JB, Purvis JA. The electrophoretic detection of acidic glycosaminoglycans in human gingival sulcus fluid. Arch Oral Biol. 1982;27:177‐179. - PubMed
-
- Pogrel MA, Low MA, Stern R. Hyaluronan (hyaluronic acid) and its regulation in human saliva by hyaluronidase and its inhibitors. J Oral Sci. 2003;45:85‐91. - PubMed
-
- Embery G, Waddington RJ, Hall RC, Last KS. Connective tissue elements as diagnostic aids in periodontology. Periodontol 2000. 2000;24:193‐214. - PubMed
-
- Ohno S, Ijuin C, Doi T, Yoneno K, Tanne K. Expression and activity of hyaluronidase in human periodontal ligament fibroblasts. J Periodontol. 2002;73:1331‐1337. - PubMed
-
- Bartold PM, Wiebkin OW, Thonard JC. Glycosaminoglycans of human gingival epithelium and connective tissue. Connect Tissue Res. 1981;9:99‐106. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

