Brillouin spectroscopy and radiography for assessment of viscoelastic and regenerative properties of mammalian bones
- PMID: 30264554
- PMCID: PMC8357194
- DOI: 10.1117/1.JBO.23.9.097004
Brillouin spectroscopy and radiography for assessment of viscoelastic and regenerative properties of mammalian bones
Abstract
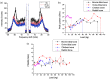
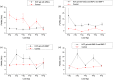
Biomechanical properties of mammalian bones, such as strength, toughness, and plasticity, are essential for understanding how microscopic-scale mechanical features can link to macroscale bones' strength and fracture resistance. We employ Brillouin light scattering (BLS) microspectroscopy for local assessment of elastic properties of bones under compression and the efficacy of the tissue engineering approach based on heparin-conjugated fibrin (HCF) hydrogels, bone morphogenic proteins, and osteogenic stem cells in the regeneration of the bone tissues. BLS is noninvasive and label-free modality for probing viscoelastic properties of tissues that can give information on structure-function properties of normal and pathological tissues. Results showed that MCS and BPMs are critically important for regeneration of elastic and viscous properties, respectively, HCF gels containing combination of all factors had the best effect with complete defect regeneration at week nine after the implantation of bone grafts and that the bones with fully consolidated fractures have higher values of elastic moduli compared with defective bones.
Keywords: Brillouin light scattering; biomechanical properties; bone morphogenic proteins; bones; critical-sized defect; elastic; heparin-conjugated fibrin gel; viscous.
(2018) COPYRIGHT Society of Photo-Optical Instrumentation Engineers (SPIE).
Figures





Similar articles
-
Brillouin Spectroscopy: From Biomedical Research to New Generation Pathology Diagnosis.Int J Mol Sci. 2021 Jul 28;22(15):8055. doi: 10.3390/ijms22158055. Int J Mol Sci. 2021. PMID: 34360822 Free PMC article. Review.
-
In vivo evaluation of MMP sensitive high-molecular weight HA-based hydrogels for bone tissue engineering.J Biomed Mater Res A. 2010 Dec 1;95(3):673-81. doi: 10.1002/jbm.a.32884. J Biomed Mater Res A. 2010. PMID: 20725983
-
Biomechanics of fibrous proteins of the extracellular matrix studied by Brillouin scattering.J R Soc Interface. 2014 Dec 6;11(101):20140739. doi: 10.1098/rsif.2014.0739. J R Soc Interface. 2014. PMID: 25297313 Free PMC article.
-
Birefringence-induced phase delay enables Brillouin mechanical imaging in turbid media.Nat Commun. 2024 Jun 19;15(1):5202. doi: 10.1038/s41467-024-49419-2. Nat Commun. 2024. PMID: 38898004 Free PMC article.
-
[Bone quantitative ultrasound].Clin Calcium. 2016 Jan;26(1):57-64. Clin Calcium. 2016. PMID: 26728531 Review. Japanese.
Cited by
-
Brillouin microscopy.Nat Rev Methods Primers. 2024;4:8. doi: 10.1038/s43586-023-00286-z. Epub 2024 Feb 1. Nat Rev Methods Primers. 2024. PMID: 39391288 Free PMC article.
-
Thermo-Visco-Elastometry of RF-Wave-Heated and Ablated Flesh Tissues Containing Au Nanoparticles.Biosensors (Basel). 2022 Dec 22;13(1):8. doi: 10.3390/bios13010008. Biosensors (Basel). 2022. PMID: 36671844 Free PMC article.
-
Brillouin Biosensing of Viscoelasticity across Phase Transitions in Ovine Cornea.Biosensors (Basel). 2024 Jul 30;14(8):371. doi: 10.3390/bios14080371. Biosensors (Basel). 2024. PMID: 39194600 Free PMC article.
-
Brillouin Spectroscopy: From Biomedical Research to New Generation Pathology Diagnosis.Int J Mol Sci. 2021 Jul 28;22(15):8055. doi: 10.3390/ijms22158055. Int J Mol Sci. 2021. PMID: 34360822 Free PMC article. Review.
-
Acoustic, Phononic, Brillouin Light Scattering and Faraday Wave-Based Frequency Combs: Physical Foundations and Applications.Sensors (Basel). 2022 May 22;22(10):3921. doi: 10.3390/s22103921. Sensors (Basel). 2022. PMID: 35632330 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

