Functional and Neuroanatomical Bases of Developmental Stuttering: Current Insights
- PMID: 30264661
- PMCID: PMC6486457
- DOI: 10.1177/1073858418803594
Functional and Neuroanatomical Bases of Developmental Stuttering: Current Insights
Abstract
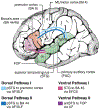
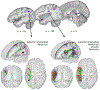
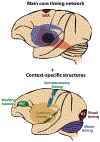
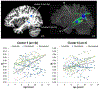
Affecting 5% of all preschool-aged children and 1% of the general population, developmental stuttering-also called childhood-onset fluency disorder-is a complex, multifactorial neurodevelopmental disorder characterized by frequent disruption of the fluent flow of speech. Over the past two decades, neuroimaging studies of both children and adults who stutter have begun to provide significant insights into the neurobiological bases of stuttering. This review highlights convergent findings from this body of literature with a focus on functional and structural neuroimaging results that are supported by theoretically driven neurocomputational models of speech production. Updated views on possible mechanisms of stuttering onset and persistence, and perspectives on promising areas for future research into the mechanisms of stuttering, are discussed.
Keywords: DTI; MRI; neurodevelopmental disorder; speech; stuttering.
Figures
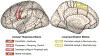

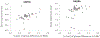
References
-
- Adams MR, Ramig P. 1980. Vocal characteristics of normal speakers and stutterers during choral reading. J Speech Hear Res 23:457–469. - PubMed
-
- Alm PA. 2004. Stuttering and the basal ganglia circuits: a critical review of possible relations. Journal of Communication Disorders 37:325–370. - PubMed
-
- Andrews CC, O’Brian SS, Harrison EE, Onslow MM, Packman AA, Menzies RR. 2012. Syllable-timed speech treatment for school-age children who stutter: a phase I trial. Language, Speech, and Hearing Services in Schools 43:359–369. - PubMed
-
- Andrews G, Harris M. 1964. The syndrome of stuttering. Spastics Society Medical Education:191.
-
- Bartolo R, Merchant H. 2015. β oscillations are linked to the initiation of sensory-cued movement sequences and the internal guidance of regular tapping in the monkey. J Neurosci [Internet] 35:4635–4640. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=β+oscillations+are+linked+to+th.... - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

