Long-term safety outcome of systemic immunosuppression in pig-to-nonhuman primate corneal xenotransplantation
- PMID: 30264877
- PMCID: PMC6166667
- DOI: 10.1111/xen.12442
Long-term safety outcome of systemic immunosuppression in pig-to-nonhuman primate corneal xenotransplantation
Abstract
Background: Safety concerns exist for corneal recipients under immunosuppression. We report long-term safety results of porcine corneal xenotransplantation under immunosuppression in nonhuman primates.
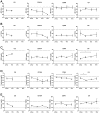
Methods: Systemic monitoring data from 49 Chinese rhesus macaques that received pig corneal transplant between 2009 and 2018 were retrospectively reviewed. The recipients were divided into 4 groups depending on the systemic immunosuppressants used: (a) conventional steroid group; costimulation blockade groups ([b] anti-CD154 antibody, [c] anti-CD40 antibody); and (d) commercially available immunosuppressants (anti-CD20 antibody, tacrolimus, basiliximab) group. We compared results of general condition monitoring; hematologic, biochemical, and electrolyte tests; and Rhesus Cytomegalovirus infection monitoring.
Results: All recipients recovered from early weight loss. White blood cell counts significantly decreased at 6 months in the steroid and anti-CD154 groups. Abnormal liver and kidney function and electrolyte imbalance were not observed in all groups. The mean value of Rhesus Cytomegalovirus DNA copies was consistently lower than 200 copies/mL, and antibody titers did not change over time in all groups. Tacrolimus-associated thrombotic microangiopathy was developed in one case, which resolved after discontinuation of tacrolimus. In 2017, a simian varicella virus outbreak led to clinical signs in 5 that received immunosuppressive therapies, of which 3 died.
Conclusion: Costimulatory blockade-based and anti-CD20 antibody/tacrolimus-based immunosuppressive therapies seem to be comparably safe with steroid therapy in nonhuman primates receiving corneal xenotransplantation, as they did not reactivate Rhesus Cytomegalovirus and maintained manageable systemic status. Although reactivation is rare, antiviral prophylaxis for simian varicella virus should be considered in immunocompromised hosts.
Keywords: cornea; immunosuppression; infection; nonhuman primates; rhesus cytomegalovirus; safety; simian varicella virus; xenotransplantation.
© 2018 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors of this manuscript disclose that there are no conflicts of interest as described by the
Figures
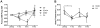


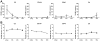
Similar articles
-
Pre-clinical results in pig-to-non-human primate islet xenotransplantation using anti-CD40 antibody (2C10R4)-based immunosuppression.Xenotransplantation. 2018 Jan;25(1):10.1111/xen.12356. doi: 10.1111/xen.12356. Epub 2017 Oct 22. Xenotransplantation. 2018. PMID: 29057561 Free PMC article.
-
Anti-CD40 antibody-mediated costimulation blockade promotes long-term survival of deep-lamellar porcine corneal grafts in non-human primates.Xenotransplantation. 2017 May;24(3):10.1111/xen.12298. doi: 10.1111/xen.12298. Epub 2017 Apr 10. Xenotransplantation. 2017. PMID: 28393447 Free PMC article.
-
The effect of epitope-based ligation of ICAM-1 on survival and retransplantation of pig islets in nonhuman primates.Xenotransplantation. 2018 Jan;25(1). doi: 10.1111/xen.12362. Epub 2017 Nov 12. Xenotransplantation. 2018. PMID: 29131413
-
Islet xenotransplantation: current status of preclinical studies in the pig-to-nonhuman primate model.Curr Opin Organ Transplant. 2008 Apr;13(2):155-8. doi: 10.1097/MOT.0b013e3282f97842. Curr Opin Organ Transplant. 2008. PMID: 18685296 Review.
-
Minimizing immunosuppression in islet xenotransplantation.Immunotherapy. 2014;6(4):419-30. doi: 10.2217/imt.14.14. Immunotherapy. 2014. PMID: 24815782 Review.
Cited by
-
Proteomics Analysis of Aqueous Humor and Rejected Graft in Pig-to-Non-Human Primate Corneal Xenotransplantation.Front Immunol. 2022 Mar 24;13:859929. doi: 10.3389/fimmu.2022.859929. eCollection 2022. Front Immunol. 2022. PMID: 35401527 Free PMC article.
-
Corneal xenotransplantation: Where are we standing?Prog Retin Eye Res. 2021 Jan;80:100876. doi: 10.1016/j.preteyeres.2020.100876. Epub 2020 Aug 2. Prog Retin Eye Res. 2021. PMID: 32755676 Free PMC article. Review.
-
Immunology in corneal transplantation-From homeostasis to graft rejection.Transplant Rev (Orlando). 2025 Apr;39(2):100909. doi: 10.1016/j.trre.2025.100909. Epub 2025 Jan 9. Transplant Rev (Orlando). 2025. PMID: 39798206 Review.
-
Predictive biomarkers for graft rejection in pig-to-non-human primate corneal xenotransplantation.Xenotransplantation. 2019 Jul;26(4):e12515. doi: 10.1111/xen.12515. Epub 2019 Apr 14. Xenotransplantation. 2019. PMID: 30983050 Free PMC article.
-
Anti-viral efficacy of a next-generation CD4-binding site bNAb in SHIV-infected animals in the absence of anti-drug antibody responses.iScience. 2022 Sep 5;25(10):105067. doi: 10.1016/j.isci.2022.105067. eCollection 2022 Oct 21. iScience. 2022. PMID: 36157588 Free PMC article.
References
-
- Lai L, Kolber-Simonds D, Park KW, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. - PubMed
-
- Yang L, Guell M, Niu D, et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs) Science. 2015;350:1101–1104. - PubMed
-
- Choi HJ, Lee JJ, Kim DH, et al. Blockade of CD40–CD154costimulatory pathway promotes long-term survival of full-thickness porcine corneal grafts in nonhuman primates: clinically applicable xenocorneal transplantation. Am J Transplant. 2015;15:628–641. - PubMed
-
- Kim MK, Hara H. Current status of corneal xenotransplantation. Int J Surg. 2015;23:255–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

