Pathogen elimination and prevention within a regulated, Designated Pathogen Free, closed pig herd for long-term breeding and production of xenotransplantation materials
- PMID: 30264879
- PMCID: PMC7169735
- DOI: 10.1111/xen.12428
Pathogen elimination and prevention within a regulated, Designated Pathogen Free, closed pig herd for long-term breeding and production of xenotransplantation materials
Abstract
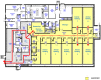
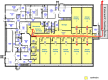
Background: We established a Source Animal (barrier) Facility (SAF) for generating designated pathogen-free (DPF) pigs to serve as donors of viable organs, tissues, or cells for xenotransplantation into clinical patients. This facility was populated with caesarian derived, colostrum deprived (CDCD) piglets, from sows of conventional-specific (or specified) pathogen-free (SPF) health status in six cohorts over a 10-month period. In all cases, CDCD piglets fulfilled DPF status including negativity for porcine circovirus (PCV), a particularly environmentally robust and difficult to inactivate virus which at the time of SAF population was epidemic in the US commercial swine production industry. Two outbreaks of PCV infection were subsequently detected during sentinel testing. The first occurred several weeks after PCV-negative animals were moved under quarantine from the nursery into an animal holding room. The apparent origin of PCV was newly installed stainless steel penning, which was not sufficiently degreased thereby protecting viral particles from disinfection. The second outbreak was apparently transmitted via employee activities in the Caesarian-section suite adjacent to the barrier facility. In both cases, PCV was contained in the animal holding room where it was diagnosed making a complete facility depopulation-repopulation unnecessary.
Method: Infectious PCV was eliminated during both outbreaks by the following: euthanizing infected animals, disposing of all removable items from the affected animal holding room, extensive cleaning with detergents and degreasing agents, sterilization of equipment and rooms with chlorine dioxide, vaporized hydrogen peroxide, and potassium peroxymonosulfate, and for the second outbreak also glutaraldehyde/quaternary ammonium. Impact on other barrier animals throughout the process was monitored by frequent PCV diagnostic testing.
Result: After close monitoring for 6 months indicating PCV absence from all rooms and animals, herd animals were removed from quarantine status.
Conclusion: Ten years after PCV clearance following the second outbreak, due to strict adherence to biosecurity protocols and based on ongoing sentinel diagnostic monitoring (currently monthly), the herd remains DPF including PCV negative.
Keywords: DPF; designated pathogen free herd; porcine circovirus; source animal facility; swine; xenotransplantation.
© 2018 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Exudative epidermitis and porcine circovirus-2 infection in a Swedish SPF-herd.Vet Microbiol. 2002 May 24;86(4):281-93. doi: 10.1016/s0378-1135(02)00024-x. Vet Microbiol. 2002. PMID: 11955778 Free PMC article.
-
Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with porcine circovirus-2 (PCV-2).Vet Pathol. 2001 Jan;38(1):31-42. doi: 10.1354/vp.38-1-31. Vet Pathol. 2001. PMID: 11199162
-
Evaluation of the transmission of porcine circovirus type 2 (PCV-2) genogroups a and b with semen from infected specific-pathogen-free boars.Vet Microbiol. 2013 Mar 23;162(2-4):381-387. doi: 10.1016/j.vetmic.2012.10.011. Epub 2012 Oct 16. Vet Microbiol. 2013. PMID: 23121716
-
Possible risks posed by single-stranded DNA viruses of pigs associated with xenotransplantation.Xenotransplantation. 2018 Jul;25(4):e12453. doi: 10.1111/xen.12453. Xenotransplantation. 2018. PMID: 30264878 Free PMC article. Review.
-
Postweaning multisystemic wasting syndrome (PMWS) in pigs with particular emphasis on the causative agent, the mode of transmission, the diagnostic tools and the control measures. A review.Vet Q. 2005 Sep;27(3):105-16. doi: 10.1080/01652176.2005.9695191. Vet Q. 2005. PMID: 16238110 Review.
Cited by
-
Surveillance and prevention of infection in clinical xenotransplantation.Clin Microbiol Rev. 2025 Mar 13;38(1):e0015023. doi: 10.1128/cmr.00150-23. Epub 2025 Jan 31. Clin Microbiol Rev. 2025. PMID: 39887237 Review.
-
Mitigating Cross-Species Viral Infections in Xenotransplantation: Progress, Strategies, and Clinical Outlook.Cell Transplant. 2024 Jan-Dec;33:9636897241226849. doi: 10.1177/09636897241226849. Cell Transplant. 2024. PMID: 38258759 Free PMC article. Review.
-
Sildenafil Citrate Enhances Renal Organogenesis Following Metanephroi Allotransplantation into Non-Immunosuppressed Hosts.J Clin Med. 2022 May 29;11(11):3068. doi: 10.3390/jcm11113068. J Clin Med. 2022. PMID: 35683456 Free PMC article.
-
Routine cold storage leads to hyperacute graft loss in pig-to-primate kidney xenotransplantation; hypothermic machine perfusion may be preferred preservation modality in xenotransplantation.Res Sq [Preprint]. 2024 Dec 18:rs.3.rs-5220149. doi: 10.21203/rs.3.rs-5220149/v1. Res Sq. 2024. Update in: Commun Med (Lond). 2025 Apr 15;5(1):117. doi: 10.1038/s43856-025-00842-6. PMID: 39764145 Free PMC article. Updated. Preprint.
-
Virus Safety of Xenotransplantation.Viruses. 2022 Aug 30;14(9):1926. doi: 10.3390/v14091926. Viruses. 2022. PMID: 36146732 Free PMC article. Review.
References
-
- Bloom ET. Xenotransplantation – federal regulatory considerations. Curr Top Microbiol Immunol. 2003;278:239‐251. - PubMed
-
- Yang L, Güell M, Niu D, et al. Genome‐wide inactivation of porcine endogenous retroviruses (PERVs). Science. 2015;350:1101‐1104. - PubMed
-
- Rosner F. Pig organs for transplantation into humans: a jewish view. Mt Sinai J Med. 1999;66:314‐319. - PubMed
-
- Vanderpool H. Pontifica academia proVita, prospects for xenotransplantation: scientific aspects and ethical considerations* (Vatican City, 2001): a critical review. Xenotransplantation. 2002;9:161‐163. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

