Protective effect of docosahexaenoic acid on lipotoxicity-mediated cell death in Schwann cells: Implication of PI3K/AKT and mTORC2 pathways
- PMID: 30264903
- PMCID: PMC6236228
- DOI: 10.1002/brb3.1123
Protective effect of docosahexaenoic acid on lipotoxicity-mediated cell death in Schwann cells: Implication of PI3K/AKT and mTORC2 pathways
Abstract
Background and aim: Docosahexaenoic acid (DHA) exhibits neuroprotective properties and has been shown to preserve nerve cells following trauma and ischemic injury. Recently, we showed that DHA pretreatment improved locomotion and reduced neuropathic pain after acute spinal cord injury in adult rats. These improvements were associated with an increase in the levels of AKT in spinal cord injury neurons. In this study, we investigate the implication of PI3K/AKT and mTOR pathway in DHA-mediated protection of primary cultured Schwann cells (pSC) undergoing palmitic acid-induced lipotoxicity (PA-LTx).
Methods: Primary cultured Schwann cells were treated with PA (PA:BSA, 2:1) in the presence or absence of DHA (1-200 µM) for 24-48 hr. Cell viability was determined by crystal violet staining and nuclear morphology was examined using Hoechst staining.
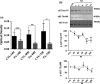
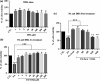
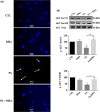
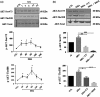
Results: We found that pSC cultures exposed to palmitic acid (PA) overload showed chromatin condensation, a decrease in cell viability and an inhibition of AKT phosphorylation in a time-dependent manner. Next, pSC exposed to PA overload were treated with DHA. The data show that co-treatment with DHA inhibited the loss of cell viability and apoptosis caused by PA. Moreover, treatment with DHA inhibited chromatin condensation, significantly stimulated p-AKT phosphorylation under PA-LTx condition, and DHA alone increased AKT phosphorylation. Additionally, when these pSC cultures were treated with PI3K inhibitors LY294002 and, BKM120 and mTOR inhibitors Torin 1 (mTORC1/mTORC2), but not rapamycin (mTORC1), the protective effects of DHA were not observed.
Conclusion: These findings suggest PI3K/AKT and mTORC2 kinase pathways are involved in the protective function (s) of DHA in PA-induced Schwann cell death.
Keywords: AKT phosphorylation; PA-induced lipotoxicity; docosahexaenoic acid; primary cultured Schwann cells.
© 2018 The Authors. Brain and Behavior published by Wiley Periodicals, Inc.
Figures


Similar articles
-
Astaxanthin and Docosahexaenoic Acid Reverse the Toxicity of the Maxi-K (BK) Channel Antagonist Mycotoxin Penitrem A.Mar Drugs. 2016 Nov 9;14(11):208. doi: 10.3390/md14110208. Mar Drugs. 2016. PMID: 27834847 Free PMC article.
-
RES-529: a PI3K/AKT/mTOR pathway inhibitor that dissociates the mTORC1 and mTORC2 complexes.Anticancer Drugs. 2016 Jul;27(6):475-87. doi: 10.1097/CAD.0000000000000354. Anticancer Drugs. 2016. PMID: 26918392 Free PMC article. Review.
-
DNMT1, a Novel Regulator Mediating mTORC1/mTORC2 Pathway-Induced NGF Expression in Schwann Cells.Neurochem Res. 2018 Nov;43(11):2141-2154. doi: 10.1007/s11064-018-2637-1. Epub 2018 Sep 18. Neurochem Res. 2018. PMID: 30229399
-
Effect of docosahexaenoic acid on hippocampal neurons in high-glucose condition: involvement of PI3K/AKT/nuclear factor-κB-mediated inflammatory pathways.Neuroscience. 2014 Aug 22;274:218-28. doi: 10.1016/j.neuroscience.2014.05.042. Epub 2014 May 29. Neuroscience. 2014. PMID: 24881575
-
Discrete signaling mechanisms of mTORC1 and mTORC2: Connected yet apart in cellular and molecular aspects.Adv Biol Regul. 2017 May;64:39-48. doi: 10.1016/j.jbior.2016.12.001. Epub 2017 Jan 4. Adv Biol Regul. 2017. PMID: 28189457 Review.
Cited by
-
Dietary Omega-3 Polyunsaturated Fatty-Acid Supplementation Upregulates Protective Cellular Pathways in Patients with Type 2 Diabetes Exhibiting Improvement in Painful Diabetic Neuropathy.Nutrients. 2022 Feb 11;14(4):761. doi: 10.3390/nu14040761. Nutrients. 2022. PMID: 35215418 Free PMC article.
-
The Influence of Nucleoside Reverse Transcriptase Inhibitors on Mitochondrial Activity, Lipid Content, and Fatty-Acid-Binding Protein Levels in Microglial HMC3 Cells.Pharmaceuticals (Basel). 2023 Nov 29;16(12):1661. doi: 10.3390/ph16121661. Pharmaceuticals (Basel). 2023. PMID: 38139788 Free PMC article.
-
Loss of Glutamate Decarboxylase 67 in Somatostatin-Expressing Neurons Leads to Anxiety-Like Behavior and Alteration in the Akt/GSK3β Signaling Pathway.Front Behav Neurosci. 2019 Jun 18;13:131. doi: 10.3389/fnbeh.2019.00131. eCollection 2019. Front Behav Neurosci. 2019. PMID: 31275123 Free PMC article.
-
Investigating the Role of Non-Coding RNA in Non-Alcoholic Fatty Liver Disease.Noncoding RNA. 2024 Jan 31;10(1):10. doi: 10.3390/ncrna10010010. Noncoding RNA. 2024. PMID: 38392965 Free PMC article. Review.
-
The stress-Wnt-signaling axis: a hypothesis for attention-deficit hyperactivity disorder and therapy approaches.Transl Psychiatry. 2020 Sep 18;10(1):315. doi: 10.1038/s41398-020-00999-9. Transl Psychiatry. 2020. PMID: 32948744 Free PMC article. Review.
References
-
- Akbar, M. , & Kim, H. Y. (2002). Protective effects of docosahexaenoic acid in staurosporine‐indce apoptosis: involvement of phosphatidylinositol‐3 kinase pathway. Journal of Neurochemistry, 82(3), 655–665. - PubMed
-
- Almaguel, F. G. , Liu, J. W. , Pacheco, F. J. , De Leon, D. , Casiano, C. A. , & De Leon, M. (2010). Lipotoxicity‐mediated cell dysfunction and death involve lysosomal membrane permeabilization and cathepsin L activity. Brain Research, 1318, 133–143. 10.1016/j.brainres.2009.12.038 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

