Acylated and unacylated ghrelin directly regulate ß-3 stimulated lipid turnover in rodent subcutaneous and visceral adipose tissue ex vivo but not in vivo
- PMID: 30265180
- PMCID: PMC6768250
- DOI: 10.1080/21623945.2018.1528811
Acylated and unacylated ghrelin directly regulate ß-3 stimulated lipid turnover in rodent subcutaneous and visceral adipose tissue ex vivo but not in vivo
Abstract
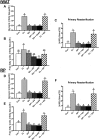
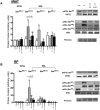
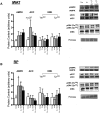
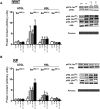
Ghrelin has garnered interest as a gut-derived regulator of lipid metabolism, beyond its classical roles in driving appetite and growth hormone release. Ghrelin's circulating concentrations follow an ultradian rhythm, peak immediately before a meal and point towards a potential metabolic role in reducing the mobilization of fatty acid stores in preparation for the storage of ingested food. Here, we demonstrate that both acylated and unacylated ghrelin have physiological roles in attenuating lipolysis in mature subcutaneous and visceral adipose tissue depots of rats. Ghrelin blunted the ß3-induction (CL 316, 243) of glycerol release (index of lipolysis) which coincided with a reduced activation of the key lipid hydrolase HSL at two of its serine residues (Ser563/660). Furthermore, ghrelin appeared to inhibit fatty acid reesterification in the presence of CL such that fatty acid concentrations in the surrounding media were maintained in spite of a reduction in lipolysis. Importantly, these aforementioned effects were not observed following ghrelin injection in vivo, as there was no attenuation of CL-induced glycerol release. This highlights the importance of exercising caution when interpreting the effects of administering ghrelin in vivo, and the necessity for uncovering the elusive mechanisms by which ghrelin regulates lipolysis and fatty acid reesterification. We conclude that both acylated and unacylated ghrelin can exert direct inhibitory effects on lipolysis and fatty acid reesterification in adipose tissue from rats. However, these effects are not observed in vivo and outline the complexity of studying ghrelin's effects on fatty acid metabolism in the living animal.
Keywords: adrenergic; endocrinology; fatty acids; growth hormone; lipolysis; metabolism; orexigenic; organ culture; reesterification; signaling.
Figures



Similar articles
-
Regulation of adipose tissue lipolysis by ghrelin is impaired with high-fat diet feeding and is not restored with exercise.Adipocyte. 2021 Dec;10(1):338-349. doi: 10.1080/21623945.2021.1945787. Adipocyte. 2021. PMID: 34224298 Free PMC article.
-
Ghrelin stimulates fatty acid oxidation and inhibits lipolysis in isolated muscle from male rats.Physiol Rep. 2019 Apr;7(7):e14028. doi: 10.14814/phy2.14028. Physiol Rep. 2019. PMID: 30963694 Free PMC article.
-
Regulation of adipose tissue and skeletal muscle substrate metabolism by the stomach-derived hormone, ghrelin.Curr Opin Pharmacol. 2020 Jun;52:25-32. doi: 10.1016/j.coph.2020.04.005. Epub 2020 May 29. Curr Opin Pharmacol. 2020. PMID: 32480033 Review.
-
Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention.Mol Endocrinol. 2009 Jun;23(6):914-24. doi: 10.1210/me.2008-0432. Epub 2009 Mar 19. Mol Endocrinol. 2009. PMID: 19299444 Free PMC article.
-
Ghrelin forms in the modulation of energy balance and metabolism.Eat Weight Disord. 2019 Dec;24(6):997-1013. doi: 10.1007/s40519-018-0599-6. Epub 2018 Oct 24. Eat Weight Disord. 2019. PMID: 30353455 Review.
Cited by
-
Regulation of adipose tissue lipolysis by ghrelin is impaired with high-fat diet feeding and is not restored with exercise.Adipocyte. 2021 Dec;10(1):338-349. doi: 10.1080/21623945.2021.1945787. Adipocyte. 2021. PMID: 34224298 Free PMC article.
-
Regulation of peripheral tissue substrate metabolism by the gut-derived hormone ghrelin.Metabol Open. 2024 Feb 28;21:100279. doi: 10.1016/j.metop.2024.100279. eCollection 2024 Mar. Metabol Open. 2024. PMID: 38487670 Free PMC article. Review.
-
Ghrelin stimulates fatty acid oxidation and inhibits lipolysis in isolated muscle from male rats.Physiol Rep. 2019 Apr;7(7):e14028. doi: 10.14814/phy2.14028. Physiol Rep. 2019. PMID: 30963694 Free PMC article.
-
Ghrelin, via corticotropin-releasing factor receptors, reduces glucose uptake and increases lipid content in mouse myoblasts cells.Physiol Rep. 2021 Jan;9(2):e14654. doi: 10.14814/phy2.14654. Physiol Rep. 2021. PMID: 33463908 Free PMC article.
-
Unacylated ghrelin stimulates fatty acid oxidation to protect skeletal muscle against palmitate-induced impairment of insulin action in lean but not high-fat fed rats.Metabol Open. 2020 Feb 1;5:100026. doi: 10.1016/j.metop.2020.100026. eCollection 2020 Mar. Metabol Open. 2020. PMID: 32812929 Free PMC article.
References
-
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. - PubMed
-
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DGA, Ghatei MA, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. - PubMed
-
- Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G.. Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:228–235. - PubMed
-
- Vestergaard ET, Djurhuus CB, Gjedsted J, Nielsen S, Møller N, Holst JJ, Jørgensen JOL, Schmitz O. Acute effects of ghrelin administration on glucose and lipid metabolism. J Clin Endocrinol Metab. 2008;93:438–444. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
