Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice
- PMID: 30265241
- PMCID: PMC6167054
- DOI: 10.7554/eLife.39636
Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice
Abstract
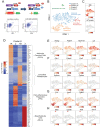
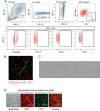
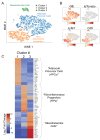
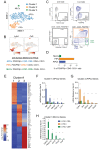
White adipose tissue (WAT) remodeling is dictated by coordinated interactions between adipocytes and resident stromal-vascular cells; however, the functional heterogeneity of adipose stromal cells has remained unresolved. We combined single-cell RNA-sequencing and FACS to identify and isolate functionally distinct subpopulations of PDGFRβ+ stromal cells within visceral WAT of adult mice. LY6C- CD9- PDGFRβ+ cells represent highly adipogenic visceral adipocyte precursor cells ('APCs'), whereas LY6C+ PDGFRβ+ cells represent fibro-inflammatory progenitors ('FIPs'). FIPs lack adipogenic capacity, display pro-fibrogenic/pro-inflammatory phenotypes, and can exert an anti-adipogenic effect on APCs. The pro-inflammatory phenotype of PDGFRβ+ cells is regulated, at least in part, by NR4A nuclear receptors. These data highlight the functional heterogeneity of visceral WAT perivascular cells, and provide insight into potential cell-cell interactions impacting adipogenesis and inflammation. These improved strategies to isolate FIPs and APCs from visceral WAT will facilitate the study of physiological WAT remodeling and mechanisms leading to metabolic dysfunction.
Editorial note: This article has been through an editorial process in which the authors decide how to respond to the issues raised during peer review. The Reviewing Editor's assessment is that all the issues have been addressed.
Keywords: adipogenesis; adipose tissue; fibrosis; human biology; inflammation; medicine; mouse; mural cells; obesity.
© 2018, Hepler et al.
Conflict of interest statement
CH, BS, QZ, GH, MS, LV, AG, AM, DS, GH, RG No competing interests declared
Figures








References
-
- Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schürmann A, Saraiva LR, Schulz TJ. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem Cell-Based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20:771–784. doi: 10.1016/j.stem.2017.02.009. - DOI - PMC - PubMed
-
- Carrière A, Jeanson Y, Côté JA, Dromard C, Galinier A, Menzel S, Barreau C, Dupuis-Coronas S, Arnaud E, Girousse A, Cuminetti V, Paupert J, Cousin B, Sengenes C, Koch-Nolte F, Tchernof A, Casteilla L. Identification of the ectoenzyme CD38 as a marker of committed preadipocytes. International Journal of Obesity. 2017;41:1539–1546. doi: 10.1038/ijo.2017.140. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- I-1926-20170325/Welch Foundation/International
- 16POST26420136/American Heart Association/International
- DP2GM128203/GM/NIGMS NIH HHS/United States
- DP2 GM128203/GM/NIGMS NIH HHS/United States
- R03 DK110497/DK/NIDDK NIH HHS/United States
- DK110497/NH/NIH HHS/United States
- F31 DK113696/DK/NIDDK NIH HHS/United States
- T32 GM008203/GM/NIGMS NIH HHS/United States
- K01 DK098277/DK/NIDDK NIH HHS/United States
- R01 DK104789/DK/NIDDK NIH HHS/United States
- RR140023/Cancer Prevention and Research Institute of Texas/International
- DK104789/NH/NIH HHS/United States
- F31DK113696/NH/NIH HHS/United States
- DK098277/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

