Passive Immunotherapies for Central Nervous System Disorders: Current Delivery Challenges and New Approaches
- PMID: 30265523
- PMCID: PMC7234797
- DOI: 10.1021/acs.bioconjchem.8b00548
Passive Immunotherapies for Central Nervous System Disorders: Current Delivery Challenges and New Approaches
Abstract
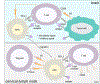
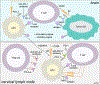
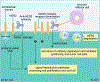
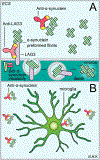
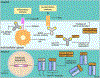
Passive immunotherapy, i.e., the administration of exogenous antibodies that recognize a specific target antigen, has gained significant momentum as a potential treatment strategy for several central nervous system (CNS) disorders, including Alzheimer's disease, Parkinson's disease, Huntington's disease, and brain cancer, among others. Advances in antibody engineering to create therapeutic antibody fragments or antibody conjugates have introduced new strategies that may also be applied to treat CNS disorders. However, drug delivery to the CNS for antibodies and other macromolecules has thus far proven challenging, due in large part to the blood-brain barrier and blood-cerebrospinal fluid barriers that greatly restrict transport of peripherally administered molecules from the systemic circulation into the CNS. Here, we summarize the various passive immunotherapy approaches under study for the treatment of CNS disorders, with a primary focus on disease-specific and target site-specific challenges to drug delivery and new, cutting edge methods.
Figures
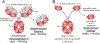





References
-
- Kindt TJ, Goldsby RA & Osborne BA Kuby Immunology. 6th Ed edn, (W. H. Freeman and Co., 2007).
-
- Köhler G. & Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

