Assessment of HIV transfusion transmission risk in South Africa: a 10-year analysis following implementation of individual donation nucleic acid amplification technology testing and donor demographics eligibility changes
- PMID: 30265757
- PMCID: PMC10419327
- DOI: 10.1111/trf.14959
Assessment of HIV transfusion transmission risk in South Africa: a 10-year analysis following implementation of individual donation nucleic acid amplification technology testing and donor demographics eligibility changes
Abstract
Background: In 1998 we estimated that 34/million infectious window period donations were entering the blood supply at the South African National Blood Service. Selective use of donations based on donor race-ethnicity reduced this risk to 26/million donations but was deemed unethical. Consequently, in 2005 South African National Blood Service eliminated race-ethnicity-based collection policies and implemented individual-donation nucleic acid testing (ID-NAT). We describe the change in donor base demographics, human immunodeficiency virus (HIV) detection rates, and transfusion-transmissible HIV risk.
Study design and methods: In ten years 7.7 million donations were tested for anti-HIV and HIV RNA. Number of donations, HIV prevalence, ID-NAT yield rate, serology yield rate and residual transfusion-transmissible HIV risk were analyzed by donor type, race-ethnicity, age, and sex. Multiple regression analysis was performed to investigate the determinants of HIV-positive and nucleic acid testing yield donations.
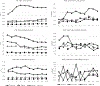
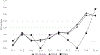
Results: The combined strategy of increasing donations from black donors and implementing ID-NAT increased the proportion of donations from black donors from 6% in 2005 to 30% in 2015 (p < 0.00001), and reduced the transfusion-transmissible risk from 24 to 13 per million transfusions. ID-NAT interdicted 481 (1:16,100) seronegative window period donations, while one transfusion-transmissible case (0.13 per million) was documented. Race-ethnicity and donor type were highly significant predictors of HIV positivity, with adjusted odds ratio for first-time donors of 12.5 (95% confidence interval, 11.9-13.1) and for black race-ethnicity of 31.1 (95% confidence interval, 28.9-33.4). The proportion of serology yields among HIV-infected donors increased from 0.27% to 2.4%.
Conclusion: ID-NAT enabled the South African National Blood Service to increase the number of donations from black donors fivefold while enhancing the safety of the blood supply.
© 2018 AABB.
Conflict of interest statement
Conflicts of interest: Nico Lelie may be paid by Grifols, the manufacturer of the NAT assay used, for review of the draft paper
There are no conflicts of interest for all other authors
Figures


Comment in
-
Technical solutions to social issues?Transfusion. 2019 Jan;59(1):9-11. doi: 10.1111/trf.15081. Transfusion. 2019. PMID: 30615812 No abstract available.
References
-
- Statistics South Africa -Mid Year population estimates. Statistical release 2017;http://www.statssa.gov.za/publications/P0302/P03022017.pdf.
-
- Heyns Adu P Risk of transmitting HIV and other diseases with a blood transfusion in South Africa. . CME (South African med assoc) 1999: 854–61.
-
- Heyns Adu P, Benjamin RJ, Swanevelder JP, Laycock ME, Pappalardo BL, Crookes RL, Wright DJ, Busch MP. Prevalence of HIV-1 in blood donations following implementation of a structured blood safety policy in South Africa. JAMA 2006;295: 519–26. - PubMed
-
- Fang CT, Field SP, Busch MP, Heyns Adu P. Human immunodeficiency virus-1 and hepatitis C virus RNA among South African blood donors: estimation of residual transfusion risk and yield of nucleic acid testing. Vox Sang 2003;85: 9–19. - PubMed
-
- Vermeulen M, Lelie N, Sykes W, Crookes R, Swanevelder J, Gaggia L, Le Roux M, Kuun E, Gulube S, Reddy R. Impact of individual-donation nucleic acid testing on risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission by blood transfusion in South Africa. Transfusion 2009;49: 1115–25. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

