Crosstalk between zinc and free fatty acids in plasma
- PMID: 30266430
- PMCID: PMC6372834
- DOI: 10.1016/j.bbalip.2018.09.007
Crosstalk between zinc and free fatty acids in plasma
Abstract
In mammalian blood plasma, serum albumin acts as a transport protein for free fatty acids, other lipids and hydrophobic molecules including neurodegenerative peptides, and essential metal ions such as zinc to allow their systemic distribution. Importantly, binding of these chemically extremely diverse entities is not independent, but linked allosterically. One particularly intriguing allosteric link exists between free fatty acid and zinc binding. Albumin thus mediates crosstalk between energy status/metabolism and organismal zinc handling. In recognition of the fact that even small changes in extracellular zinc concentration and speciation modulate the function of many cell types, the albumin-mediated impact of free fatty acid concentration on zinc distribution may be significant for both normal physiological processes including energy metabolism, insulin activity, heparin neutralisation, blood coagulation, and zinc signalling, and a range of disease states, including metabolic syndrome, cardiovascular disease, myocardial ischemia, diabetes, and thrombosis.
Keywords: Albumin; Non-esterified fatty acids; Plasma; Serum; Zinc.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



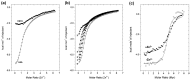
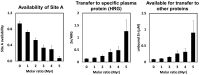
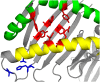
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

