Angiomotins stimulate LATS kinase autophosphorylation and act as scaffolds that promote Hippo signaling
- PMID: 30266805
- PMCID: PMC6254346
- DOI: 10.1074/jbc.RA118.004187
Angiomotins stimulate LATS kinase autophosphorylation and act as scaffolds that promote Hippo signaling
Abstract
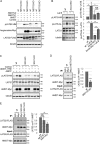
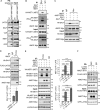
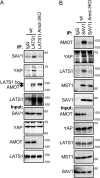
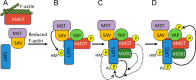
The Hippo pathway controls cell proliferation, differentiation, and survival by regulating the Yes-associated protein (YAP) transcriptional coactivator in response to various stimuli, including the mechanical environment. The major YAP regulators are the LATS1/2 kinases, which phosphorylate and inhibit YAP. LATS1/2 are activated by phosphorylation on a hydrophobic motif (HM) outside of the kinase domain by MST1/2 and other kinases. Phosphorylation of the HM motif then triggers autophosphorylation of the kinase in the activation loop to fully activate the kinase, a process facilitated by MOB1. The angiomotin family of proteins (AMOT, AMOTL1, and AMOTL2) bind LATS1/2 and promote its kinase activity and YAP phosphorylation through an unknown mechanism. Here we show that angiomotins increase Hippo signaling through multiple mechanisms. We found that, by binding LATS1/2, SAV1, and YAP, angiomotins function as a scaffold that connects LATS1/2 to both its activator SAV1-MST1 and its target YAP. Deletion of all three angiomotins reduced the association of LATS1 with SAV1-MST1 and decreased MST1/2-mediated LATS1/2-HM phosphorylation. Angiomotin deletion also reduced LATS1/2's ability to associate with and phosphorylate YAP. In addition, we found that angiomotins have an unexpected function along with MOB1 to promote autophosphorylation of LATS1/2 on the activation loop motif independent of HM phosphorylation. These results indicate that angiomotins enhance Hippo signaling by stimulating LATS1/2 autophosphorylation and by connecting LATS1/2 with both its activator SAV1-MST1/2 and its substrate YAP.
Keywords: AMOT; Hippo pathway; LATS (Warts, Wts); MOB1; SAV1; Salvador (Sav); Yes-associated protein (YAP); angiomotin; cell signaling; mammalian sterile 20-like kinase 1 (MST1); mammalian sterile 20-like kinase 2 (MST2); mechanotransduction; protein kinase; scaffold protein.
© 2018 Mana-Capelli and McCollum.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures


Similar articles
-
Role of Angiomotin-like 2 mono-ubiquitination on YAP inhibition.EMBO Rep. 2016 Jan;17(1):64-78. doi: 10.15252/embr.201540809. Epub 2015 Nov 23. EMBO Rep. 2016. PMID: 26598551 Free PMC article.
-
Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17368-73. doi: 10.1073/pnas.1308236110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101513 Free PMC article.
-
Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor.Mol Biol Cell. 2011 Oct;22(19):3725-33. doi: 10.1091/mbc.E11-04-0300. Epub 2011 Aug 10. Mol Biol Cell. 2011. PMID: 21832154 Free PMC article.
-
Angiomotin'g YAP into the nucleus for cell proliferation and cancer development.Sci Signal. 2013 Sep 3;6(291):pe27. doi: 10.1126/scisignal.2004573. Sci Signal. 2013. PMID: 24003252 Review.
-
Protein kinases of the Hippo pathway: regulation and substrates.Semin Cell Dev Biol. 2012 Sep;23(7):770-84. doi: 10.1016/j.semcdb.2012.07.002. Epub 2012 Aug 9. Semin Cell Dev Biol. 2012. PMID: 22898666 Free PMC article. Review.
Cited by
-
Tudor-SN promotes cardiomyocyte proliferation and neonatal heart regeneration through regulating the phosphorylation of YAP.Cell Commun Signal. 2024 Jun 28;22(1):345. doi: 10.1186/s12964-024-01715-6. Cell Commun Signal. 2024. PMID: 38943195 Free PMC article.
-
Pharmacological inhibition of CLK2 activates YAP by promoting alternative splicing of AMOTL2.Elife. 2023 Dec 21;12:RP88508. doi: 10.7554/eLife.88508. Elife. 2023. PMID: 38126343 Free PMC article.
-
The YAP/TAZ Pathway in Osteogenesis and Bone Sarcoma Pathogenesis.Cells. 2020 Apr 15;9(4):972. doi: 10.3390/cells9040972. Cells. 2020. PMID: 32326412 Free PMC article. Review.
-
The Hippo-YAP pathway in various cardiovascular diseases: Focusing on the inflammatory response.Front Immunol. 2022 Aug 18;13:971416. doi: 10.3389/fimmu.2022.971416. eCollection 2022. Front Immunol. 2022. PMID: 36059522 Free PMC article. Review.
-
The Hippo signalling pathway and its implications in human health and diseases.Signal Transduct Target Ther. 2022 Nov 8;7(1):376. doi: 10.1038/s41392-022-01191-9. Signal Transduct Target Ther. 2022. PMID: 36347846 Free PMC article. Review.
References
-
- Liu C. Y., Zha Z. Y., Zhou X., Zhang H., Huang W., Zhao D., Li T., Chan S. W., Lim C. J., Hong W., Zhao S., Xiong Y., Lei Q. Y., and Guan K. L. (2010) The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFβ-TrCP E3 ligase. J. Biol. Chem. 285, 37159–37169 10.1074/jbc.M110.152942 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

