Activatable probes for diagnosing and positioning liver injury and metastatic tumors by multispectral optoacoustic tomography
- PMID: 30266905
- PMCID: PMC6162313
- DOI: 10.1038/s41467-018-06499-1
Activatable probes for diagnosing and positioning liver injury and metastatic tumors by multispectral optoacoustic tomography
Abstract
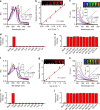
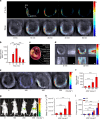
Optoacoustic tomography (photoacoustic tomography) is an emerging imaging technology displaying great potential for medical diagnosis and preclinical research. Rationally designing activatable optoacoustic probes capable of diagnosing diseases and locating their foci can bring into full play the role of optoacoustic tomography (OAT) as a promising noninvasive imaging modality. Here we report two xanthene-based optoacoustic probes (C1X-OR1 and C2X-OR2) for temporospatial imaging of hepatic alkaline phosphatase (or β-galactosidase) for evaluating and locating drug-induced liver injury (or metastatic tumor). The probes rapidly respond to the disease-specific biomarkers by displaying red-shifted NIR absorption bands and generate prominent optoacoustic signals. Using multispectral optoacoustic tomography (MSOT), we can precisely localize the focus of drug-induced liver injury in mice using C1X-OR1, and the metastatic tumors using C2X-OR2. This work suggests that the activatable optoacoustic chromophores may potentially be applied for diagnosing and localizing disease foci, especially smaller and deeper ones.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Diagnosing Drug-Induced Liver Injury by Multispectral Optoacoustic Tomography and Fluorescence Imaging Using a Leucine-Aminopeptidase-Activated Probe.Anal Chem. 2019 Jul 2;91(13):8085-8092. doi: 10.1021/acs.analchem.9b00107. Epub 2019 Jun 10. Anal Chem. 2019. PMID: 31145584
-
ALP-activated probe for diagnosis of liver injury by multispectral optoacoustic tomography.Methods Enzymol. 2021;657:301-330. doi: 10.1016/bs.mie.2021.06.019. Epub 2021 Jul 12. Methods Enzymol. 2021. PMID: 34353492
-
A H2O2-activatable nanoprobe for diagnosing interstitial cystitis and liver ischemia-reperfusion injury via multispectral optoacoustic tomography and NIR-II fluorescent imaging.Nat Commun. 2021 Nov 25;12(1):6870. doi: 10.1038/s41467-021-27233-4. Nat Commun. 2021. PMID: 34824274 Free PMC article.
-
Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology.Clin Cancer Res. 2016 Jul 15;22(14):3432-9. doi: 10.1158/1078-0432.CCR-16-0573. Epub 2016 May 20. Clin Cancer Res. 2016. PMID: 27208064 Free PMC article. Review.
-
Development of Multispectral Optoacoustic Tomography as a Clinically Translatable Modality for Cancer Imaging.Radiol Imaging Cancer. 2020 Nov 20;2(6):e200066. doi: 10.1148/rycan.2020200066. eCollection 2020 Nov. Radiol Imaging Cancer. 2020. PMID: 33330850 Free PMC article. Review.
Cited by
-
An Activatable Near-Infrared Chromophore for Multispectral Optoacoustic Imaging of Tumor Hypoxia and for Tumor Inhibition.Theranostics. 2019 Sep 25;9(24):7313-7324. doi: 10.7150/thno.36755. eCollection 2019. Theranostics. 2019. PMID: 31695770 Free PMC article.
-
Enzyme-activated near-infrared fluorogenic probe with high-efficiency intrahepatic targeting ability for visualization of drug-induced liver injury.Chem Sci. 2021 Oct 20;12(44):14855-14862. doi: 10.1039/d1sc04825b. eCollection 2021 Nov 17. Chem Sci. 2021. PMID: 34820101 Free PMC article.
-
Development of NIR-II Photoacoustic Probes Tailored for Deep-Tissue Sensing of Nitric Oxide.J Am Chem Soc. 2021 May 12;143(18):7196-7202. doi: 10.1021/jacs.1c03004. Epub 2021 Apr 27. J Am Chem Soc. 2021. PMID: 33905646 Free PMC article.
-
Technical validation studies of a dual-wavelength LED-based photoacoustic and ultrasound imaging system.Photoacoustics. 2021 Apr 10;22:100267. doi: 10.1016/j.pacs.2021.100267. eCollection 2021 Jun. Photoacoustics. 2021. PMID: 33948434 Free PMC article.
-
Targeted contrast agents and activatable probes for photoacoustic imaging of cancer.Chem Soc Rev. 2022 Feb 7;51(3):829-868. doi: 10.1039/d0cs00771d. Chem Soc Rev. 2022. PMID: 35094040 Free PMC article. Review.
References
-
- Hong S, Antaris AL, Dai H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017;1:0010. doi: 10.1038/s41551-016-0010. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

