An iris diaphragm mechanism to gate a cyclic nucleotide-gated ion channel
- PMID: 30266906
- PMCID: PMC6162275
- DOI: 10.1038/s41467-018-06414-8
An iris diaphragm mechanism to gate a cyclic nucleotide-gated ion channel
Abstract
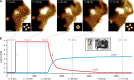
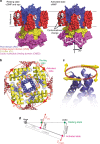
Cyclic nucleotide-gated (CNG) ion channels are non-selective cation channels key to signal transduction. The free energy difference of cyclic-nucleotide (cAMP/cGMP) binding/unbinding is translated into mechanical work to modulate the open/closed probability of the pore, i.e., gating. Despite the recent advances in structural determination of CNG channels, the conformational changes associated with gating remain unknown. Here we examine the conformational dynamics of a prokaryotic homolog of CNG channels, SthK, using high-speed atomic force microscopy (HS-AFM). HS-AFM of SthK in lipid bilayers shows that the CNBDs undergo dramatic conformational changes during the interconversion between the resting (apo and cGMP) and the activated (cAMP) states: the CNBDs approach the membrane and splay away from the 4-fold channel axis accompanied by a clockwise rotation with respect to the pore domain. We propose that these movements may be converted by the C-linker to pull the pore helices open in an iris diaphragm-like mechanism.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Ligand binding and activation properties of the purified bacterial cyclic nucleotide-gated channel SthK.J Gen Physiol. 2018 Jun 4;150(6):821-834. doi: 10.1085/jgp.201812023. Epub 2018 May 11. J Gen Physiol. 2018. PMID: 29752414 Free PMC article.
-
Structure of the SthK carboxy-terminal region reveals a gating mechanism for cyclic nucleotide-modulated ion channels.PLoS One. 2015 Jan 27;10(1):e0116369. doi: 10.1371/journal.pone.0116369. eCollection 2015. PLoS One. 2015. PMID: 25625648 Free PMC article.
-
Allosteric conformational change of a cyclic nucleotide-gated ion channel revealed by DEER spectroscopy.Proc Natl Acad Sci U S A. 2020 May 19;117(20):10839-10847. doi: 10.1073/pnas.1916375117. Epub 2020 May 1. Proc Natl Acad Sci U S A. 2020. PMID: 32358188 Free PMC article.
-
Structural snapshot of cyclic nucleotide binding domains from cyclic nucleotide-sensitive ion channels.Biol Chem. 2013 Nov;394(11):1439-51. doi: 10.1515/hsz-2013-0228. Biol Chem. 2013. PMID: 24021595 Review.
-
Cyclic nucleotide-gated channels.Handb Exp Pharmacol. 2009;(191):111-36. doi: 10.1007/978-3-540-68964-5_7. Handb Exp Pharmacol. 2009. PMID: 19089328 Review.
Cited by
-
Real time dynamics of Gating-Related conformational changes in CorA.Elife. 2019 Nov 27;8:e47322. doi: 10.7554/eLife.47322. Elife. 2019. PMID: 31774394 Free PMC article.
-
Domain coupling in allosteric regulation of SthK measured using time-resolved transition metal ion FRET.Elife. 2025 Aug 12;14:RP106892. doi: 10.7554/eLife.106892. Elife. 2025. PMID: 40792615 Free PMC article.
-
New Structures and Gating of Voltage-Dependent Potassium (Kv) Channels and Their Relatives: A Multi-Domain and Dynamic Question.Int J Mol Sci. 2019 Jan 10;20(2):248. doi: 10.3390/ijms20020248. Int J Mol Sci. 2019. PMID: 30634573 Free PMC article. Review.
-
Mechanism of ligand activation of a eukaryotic cyclic nucleotide-gated channel.Nat Struct Mol Biol. 2020 Jul;27(7):625-634. doi: 10.1038/s41594-020-0433-5. Epub 2020 Jun 1. Nat Struct Mol Biol. 2020. PMID: 32483338 Free PMC article.
-
Correlating ion channel structure and function.Methods Enzymol. 2021;652:3-30. doi: 10.1016/bs.mie.2021.02.016. Epub 2021 Mar 25. Methods Enzymol. 2021. PMID: 34059287 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01GM124451/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)/International
- 310080/ERC_/European Research Council/International
- 310080/EC | European Research Council (ERC)/International
- MSCA IF-2014-EF-655157/EC | Horizon 2020 (European Union Framework Programme for Research and Innovation)/International
- R01 GM124451/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

