Three-Dimensional Retinal Organoids Facilitate the Investigation of Retinal Ganglion Cell Development, Organization and Neurite Outgrowth from Human Pluripotent Stem Cells
- PMID: 30266927
- PMCID: PMC6162218
- DOI: 10.1038/s41598-018-32871-8
Three-Dimensional Retinal Organoids Facilitate the Investigation of Retinal Ganglion Cell Development, Organization and Neurite Outgrowth from Human Pluripotent Stem Cells
Abstract
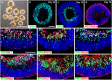
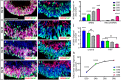
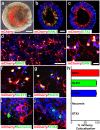
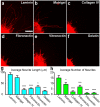
Retinal organoids are three-dimensional structures derived from human pluripotent stem cells (hPSCs) which recapitulate the spatial and temporal differentiation of the retina, serving as effective in vitro models of retinal development. However, a lack of emphasis has been placed upon the development and organization of retinal ganglion cells (RGCs) within retinal organoids. Thus, initial efforts were made to characterize RGC differentiation throughout early stages of organoid development, with a clearly defined RGC layer developing in a temporally-appropriate manner expressing a complement of RGC-associated markers. Beyond studies of RGC development, retinal organoids may also prove useful for cellular replacement in which extensive axonal outgrowth is necessary to reach post-synaptic targets. Organoid-derived RGCs could help to elucidate factors promoting axonal outgrowth, thereby identifying approaches to circumvent a formidable obstacle to RGC replacement. As such, additional efforts demonstrated significant enhancement of neurite outgrowth through modulation of both substrate composition and growth factor signaling. Additionally, organoid-derived RGCs exhibited diverse phenotypes, extending elaborate growth cones and expressing numerous guidance receptors. Collectively, these results establish retinal organoids as a valuable tool for studies of RGC development, and demonstrate the utility of organoid-derived RGCs as an effective platform to study factors influencing neurite outgrowth from organoid-derived RGCs.
Conflict of interest statement
The authors declare no competing interests.
Figures
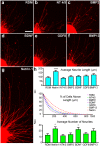
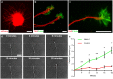
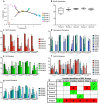
References
-
- Muramatsu R, Ueno M, Yamashita T. Intrinsic regenerative mechanisms of central nervous system neurons. Bioscience trends. 2009;3:179–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

