Structural basis of neurosteroid anesthetic action on GABAA receptors
- PMID: 30266951
- PMCID: PMC6162318
- DOI: 10.1038/s41467-018-06361-4
Structural basis of neurosteroid anesthetic action on GABAA receptors
Abstract
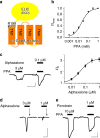
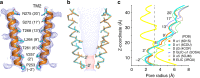
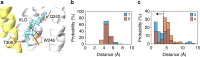
Type A γ-aminobutyric acid receptors (GABAARs) are inhibitory pentameric ligand-gated ion channels in the brain. Many anesthetics and neurosteroids act through binding to the GABAAR transmembrane domain (TMD), but the structural basis of their actions is not well understood and no resting-state GABAAR structure has been determined. Here, we report crystal structures of apo and the neurosteroid anesthetic alphaxalone-bound desensitized chimeric α1GABAAR (ELIC-α1GABAAR). The chimera retains the functional and pharmacological properties of GABAARs, including potentiation, activation and desensitization by alphaxalone. The apo-state structure reveals an unconventional activation gate at the intracellular end of the pore. The desensitized structure illustrates molecular determinants for alphaxalone binding to an inter-subunit TMD site. These structures suggest a plausible signaling pathway from alphaxalone binding at the bottom of the TMD to the channel gate in the pore-lining TM2 through the TM1-TM2 linker. The study provides a framework to discover new GABAAR modulators with therapeutic potential.
Conflict of interest statement
The authors declare no competing interests.
Figures


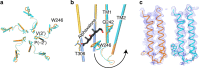
Similar articles
-
Structural basis for GABAA receptor potentiation by neurosteroids.Nat Struct Mol Biol. 2017 Nov;24(11):986-992. doi: 10.1038/nsmb.3484. Epub 2017 Oct 9. Nat Struct Mol Biol. 2017. PMID: 28991263 Free PMC article.
-
Common Anesthetic-binding Site for Inhibition of Pentameric Ligand-gated Ion Channels.Anesthesiology. 2016 Mar;124(3):664-73. doi: 10.1097/ALN.0000000000001005. Anesthesiology. 2016. PMID: 26756520 Free PMC article.
-
A chimeric prokaryotic-eukaryotic pentameric ligand gated ion channel reveals interactions between the extracellular and transmembrane domains shape neurosteroid modulation.Neuropharmacology. 2017 Oct;125:343-352. doi: 10.1016/j.neuropharm.2017.08.007. Epub 2017 Aug 10. Neuropharmacology. 2017. PMID: 28803966 Free PMC article.
-
In Search of GABAA Receptor's Neurosteroid Binding Sites.J Med Chem. 2019 Jun 13;62(11):5250-5260. doi: 10.1021/acs.jmedchem.8b01400. Epub 2018 Dec 31. J Med Chem. 2019. PMID: 30566352 Review.
-
Combining Mutations and Electrophysiology to Map Anesthetic Sites on Ligand-Gated Ion Channels.Methods Enzymol. 2018;602:369-389. doi: 10.1016/bs.mie.2018.01.014. Epub 2018 Feb 28. Methods Enzymol. 2018. PMID: 29588039 Free PMC article. Review.
Cited by
-
Epileptic Encephalopathy GABRB Structural Variants Share Common Gating and Trafficking Defects.Biomolecules. 2023 Dec 14;13(12):1790. doi: 10.3390/biom13121790. Biomolecules. 2023. PMID: 38136660 Free PMC article.
-
Alfaxalone does not have long-term effects on goldfish pyramidal neuron action potential properties or GABAA receptor currents.FEBS Open Bio. 2024 Apr;14(4):555-573. doi: 10.1002/2211-5463.13777. Epub 2024 Feb 11. FEBS Open Bio. 2024. PMID: 38342633 Free PMC article.
-
Photomotor Responses in Zebrafish and Electrophysiology Reveal Varying Interactions of Anesthetics Targeting Distinct Sites on γ-Aminobutyric Acid Type A Receptors.Anesthesiology. 2022 Nov 1;137(5):568-585. doi: 10.1097/ALN.0000000000004361. Anesthesiology. 2022. PMID: 36018576 Free PMC article.
-
Structural insights into opposing actions of neurosteroids on GABAA receptors.Nat Commun. 2023 Aug 22;14(1):5091. doi: 10.1038/s41467-023-40800-1. Nat Commun. 2023. PMID: 37607940 Free PMC article.
-
Photoaffinity labeling identifies an intersubunit steroid-binding site in heteromeric GABA type A (GABAA) receptors.J Biol Chem. 2020 Aug 14;295(33):11495-11512. doi: 10.1074/jbc.RA120.013452. Epub 2020 Jun 15. J Biol Chem. 2020. PMID: 32540960 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- T32 EB009403/EB/NIBIB NIH HHS/United States
- R01GM056257/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)/International
- R01 GM056257/GM/NIGMS NIH HHS/United States
- TG-MCB050030N/National Science Foundation (NSF)/International
- P41 GM103393/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

