GLS-409, an Antagonist of Both P2Y1 and P2Y12, Potently Inhibits Canine Coronary Artery Thrombosis and Reversibly Inhibits Human Platelet Activation
- PMID: 30266987
- PMCID: PMC6162268
- DOI: 10.1038/s41598-018-32797-1
GLS-409, an Antagonist of Both P2Y1 and P2Y12, Potently Inhibits Canine Coronary Artery Thrombosis and Reversibly Inhibits Human Platelet Activation
Erratum in
-
Publisher Correction: GLS-409, an Antagonist of Both P2Y1 and P2Y12, Potently Inhibits Canine Coronary Artery Thrombosis and Reversibly Inhibits Human Platelet Activation.Sci Rep. 2018 Nov 29;8(1):17580. doi: 10.1038/s41598-018-35956-6. Sci Rep. 2018. PMID: 30498241 Free PMC article.
Abstract
Dual antiplatelet therapy with aspirin and an adenosine diphosphate (ADP) P2Y12 receptor antagonist reduces ischemic events in patients with acute coronary syndrome. Previous evidence from our group, obtained in a preclinical model of recurrent platelet-mediated thrombosis, demonstrated that GLS-409, a diadenosine tetraphosphate derivative that inhibits both P2Y1 and P2Y12 ADP receptors, may be a novel and promising antiplatelet drug candidate. However, the salutary antiplatelet effects of GLS-409 were accompanied by a trend toward an unfavorable increase in bleeding. The goals of this study were to: 1) provide proof-of-concept that the efficacy of GLS-409 may be maintained at lower dose(s), not accompanied by an increased propensity to bleeding; and 2) establish the extent and kinetics of the reversibility of human platelet inhibition by the agent. Lower doses of GLS-409 were identified that inhibited in vivo recurrent coronary thrombosis with no increase in bleeding time. Human platelet inhibition by GLS-409 was reversible, with rapid recovery of platelet reactivity to ADP, as measured by platelet surface activated GPIIb-IIIa and platelet surface P-selectin. These data support the concept that GLS-409 warrants further, larger-scale investigation as a novel, potential therapy in acute coronary syndromes.
Conflict of interest statement
E. Smolensky Koganov has nothing to disclose. I. B. Yanachkov, M. I. Yanachkova and G. E. Wright, are employees of GLSynthesis, Inc. A. D. Michelson has received grant support from GLSynthesis and Lilly/Daiichi Sankyo, and served on a steering committee for an AstraZeneca clinical trial. A. L. Frelinger has received grant support from GLSynthesis and Lilly/Daiichi Sankyo. K. Przyklenk has received grant support from GLSynthesis and serves on the scientific advisory board of Infarct Reduction Technologies, Inc.
Figures

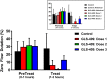

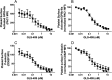

Similar articles
-
Synergistic Inhibition of Both P2Y1 and P2Y12 Adenosine Diphosphate Receptors As Novel Approach to Rapidly Attenuate Platelet-Mediated Thrombosis.Arterioscler Thromb Vasc Biol. 2016 Mar;36(3):501-9. doi: 10.1161/ATVBAHA.115.306885. Epub 2016 Jan 7. Arterioscler Thromb Vasc Biol. 2016. PMID: 26743169 Free PMC article.
-
New highly active antiplatelet agents with dual specificity for platelet P2Y1 and P2Y12 adenosine diphosphate receptors.Eur J Med Chem. 2016 Jan 1;107:204-18. doi: 10.1016/j.ejmech.2015.10.055. Epub 2015 Nov 9. Eur J Med Chem. 2016. PMID: 26588064 Free PMC article.
-
Modified diadenosine tetraphosphates with dual specificity for P2Y1 and P2Y12 are potent antagonists of ADP-induced platelet activation.J Thromb Haemost. 2012 Dec;10(12):2573-80. doi: 10.1111/jth.12035. J Thromb Haemost. 2012. PMID: 23083103 Free PMC article.
-
Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding.J Am Coll Cardiol. 2013 Dec 17;62(24):2261-73. doi: 10.1016/j.jacc.2013.07.101. Epub 2013 Sep 27. J Am Coll Cardiol. 2013. PMID: 24076493
-
Use of antiplatelet agents in sepsis: a glimpse into the future.Thromb Res. 2014 Feb;133(2):131-8. doi: 10.1016/j.thromres.2013.07.002. Epub 2013 Oct 6. Thromb Res. 2014. PMID: 24103487 Review.
Cited by
-
The Interplay of Endothelial P2Y Receptors in Cardiovascular Health: From Vascular Physiology to Pathology.Int J Mol Sci. 2022 May 24;23(11):5883. doi: 10.3390/ijms23115883. Int J Mol Sci. 2022. PMID: 35682562 Free PMC article. Review.
-
Antiplatelet Agents Affecting GPCR Signaling Implicated in Tumor Metastasis.Cells. 2022 Feb 18;11(4):725. doi: 10.3390/cells11040725. Cells. 2022. PMID: 35203374 Free PMC article. Review.
-
Platelet P2Y1 receptor exhibits constitutive G protein signaling and β-arrestin 2 recruitment.BMC Biol. 2023 Feb 1;21(1):14. doi: 10.1186/s12915-023-01528-y. BMC Biol. 2023. PMID: 36721118 Free PMC article.
-
Current concepts and novel targets for antiplatelet therapy.Nat Rev Cardiol. 2023 Sep;20(9):583-599. doi: 10.1038/s41569-023-00854-6. Epub 2023 Apr 4. Nat Rev Cardiol. 2023. PMID: 37016032 Review.
-
Ap4A in Cancer: A Multifaceted Regulator and Emerging Therapeutic Target.Molecules. 2025 Jul 22;30(15):3056. doi: 10.3390/molecules30153056. Molecules. 2025. PMID: 40807231 Free PMC article. Review.
References
-
- Amsterdam EA, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. doi: 10.1161/CIR.0000000000000133. - DOI - PubMed
-
- Hamm CW, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

