Berberine attenuates ischemia-reperfusion injury through inhibiting HMGB1 release and NF-κB nuclear translocation
- PMID: 30266998
- PMCID: PMC6289370
- DOI: 10.1038/s41401-018-0160-1
Berberine attenuates ischemia-reperfusion injury through inhibiting HMGB1 release and NF-κB nuclear translocation
Abstract
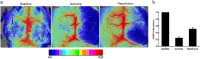
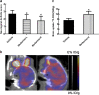
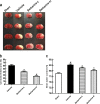
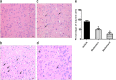
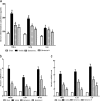
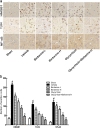
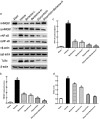
Inflammatory damage plays an important role in cerebral ischemic pathogenesis and represents a new target for treatment of stroke. Berberine is a natural medicine with multiple beneficial biological activities. In this study, we explored the mechanisms underlying the neuroprotective action of berberine in mice subjected transient middle cerebral artery occlusion (tMCAO). Male mice were administered berberine (25, 50 mg/kg/d, intragastric; i.g.), glycyrrhizin (50 mg/kg/d, intraperitoneal), or berberine (50 mg/kg/d, i.g.) plus glycyrrhizin (50 mg/kg/d, intraperitoneal) for 14 consecutive days before tMCAO. The neurological deficit scores were evaluated at 24 h after tMCAO, and then the mice were killed to obtain the brain samples. We showed that pretreatment with berberine dose-dependently decreased the infarct size, neurological deficits, hispathological changes, brain edema, and inflammatory mediators in serum and ischemic cortical tissue. We revealed that pretreatment with berberine significantly enhanced uptake of 18F-fluorodeoxyglucose of ischemic hemisphere comparing with the vehicle group at 24 h after stroke. Furthermore, pretreatment with berberine dose-dependently suppressed the nuclear-to cytosolic translocation of high-mobility group box1 (HMGB1) protein, the cytosolic-to nuclear translocation of nuclear factor kappa B (NF-κB) and decreased the expression of TLR4 in ischemic cortical tissue. Moreover, co-administration of glycyrrhizin and berberine exerted more potent suppression on the HMGB1/TLR4/NF-κB pathway than berberine or glycyrrhizin administered alone. These results demonstrate that berberine protects the brain from ischemia-reperfusion injury and the mechanism may rely on its anti-inflammatory effects mediated by suppressing the activation of HMGB1/TLR4/NF-κB signaling.
Keywords: HMGB1; NF-κB; TLR4; berberine; glycyrrhizin; inflammation; ischemic stroke.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Glycyrrhizin protects brain against ischemia-reperfusion injury in mice through HMGB1-TLR4-IL-17A signaling pathway.Brain Res. 2014 Sep 25;1582:176-86. doi: 10.1016/j.brainres.2014.07.002. Epub 2014 Aug 8. Brain Res. 2014. PMID: 25111887
-
Engeletin alleviates cerebral ischemia reperfusion-induced neuroinflammation via the HMGB1/TLR4/NF-κB network.J Cell Mol Med. 2023 Jun;27(12):1653-1663. doi: 10.1111/jcmm.17758. Epub 2023 May 2. J Cell Mol Med. 2023. PMID: 37132060 Free PMC article.
-
Daphnetin Protects against Cerebral Ischemia/Reperfusion Injury in Mice via Inhibition of TLR4/NF-κB Signaling Pathway.Biomed Res Int. 2016;2016:2816056. doi: 10.1155/2016/2816056. Epub 2016 Dec 29. Biomed Res Int. 2016. PMID: 28119924 Free PMC article.
-
Berberine protects against ischemia-reperfusion injury: A review of evidence from animal models and clinical studies.Pharmacol Res. 2019 Oct;148:104385. doi: 10.1016/j.phrs.2019.104385. Epub 2019 Aug 7. Pharmacol Res. 2019. PMID: 31400402 Review.
-
Neuroprotective effects of berberine in preclinical models of ischemic stroke: a systematic review.BMC Pharmacol Toxicol. 2025 Feb 21;26(1):40. doi: 10.1186/s40360-025-00843-0. BMC Pharmacol Toxicol. 2025. PMID: 39985090 Free PMC article.
Cited by
-
Long non-coding RNA GAS5 aggravates myocardial depression in mice with sepsis via the microRNA-449b/HMGB1 axis and the NF-κB signaling pathway.Biosci Rep. 2021 Apr 30;41(4):BSR20201738. doi: 10.1042/BSR20201738. Biosci Rep. 2021. PMID: 33645622 Free PMC article.
-
Application of Stem Cells in Stroke: A Multifactorial Approach.Front Neurosci. 2020 Jun 9;14:473. doi: 10.3389/fnins.2020.00473. eCollection 2020. Front Neurosci. 2020. PMID: 32581669 Free PMC article. Review.
-
Berberine Ameliorates Inflammation in Acute Lung Injury via NF-κB/Nlrp3 Signaling Pathway.Front Nutr. 2022 Feb 25;9:851255. doi: 10.3389/fnut.2022.851255. eCollection 2022. Front Nutr. 2022. PMID: 35284463 Free PMC article.
-
Angong Niuhuang Wan reduces hemorrhagic transformation and mortality in ischemic stroke rats with delayed thrombolysis: involvement of peroxynitrite-mediated MMP-9 activation.Chin Med. 2022 Apr 27;17(1):51. doi: 10.1186/s13020-022-00595-7. Chin Med. 2022. PMID: 35477576 Free PMC article.
-
Salvianolic Acid D Alleviates Cerebral Ischemia-Reperfusion Injury by Suppressing the Cytoplasmic Translocation and Release of HMGB1-Triggered NF-κB Activation to Inhibit Inflammatory Response.Mediators Inflamm. 2020 Jan 22;2020:9049614. doi: 10.1155/2020/9049614. eCollection 2020. Mediators Inflamm. 2020. PMID: 32410871 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

