Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia
- PMID: 30267011
- PMCID: PMC6438769
- DOI: 10.1038/s41375-018-0265-z
Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia
Erratum in
-
Correction: Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia.Leukemia. 2019 Apr;33(4):1061-1062. doi: 10.1038/s41375-019-0426-8. Leukemia. 2019. PMID: 30842605 Free PMC article.
Abstract
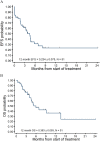
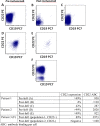
Although inotuzumab ozogamicin (InO) is recognized as an effective agent in relapsed acute lymphoblastic leukemia (ALL) in adults, data on safety and efficacy in pediatric patients are scarce. We report the use of InO in 51 children with relapsed/refractory ALL treated in the compassionate use program. In this heavily pretreated cohort, complete remission was achieved in 67% of patients with overt marrow disease. The majority (71%) of responders were negative for minimal residual disease. Responses were observed irrespective of cytogenetic subtype or number or type of prior treatment regimens. InO was well-tolerated; grade 3 hepatic transaminitis or hyperbilirubinemia were noted in 6 (12%) and grade 3/4 infections in 11 (22%) patients. No patient developed sinusoidal obstruction syndrome (SOS) during InO therapy; however, 11 of 21 (52%) patients who underwent hematopoietic stem cell transplantation (HSCT) following InO developed SOS. Downregulation of surface CD22 was detected as a possible escape mechanism in three patients who developed a subsequent relapse after InO. We conclude that InO is a well-tolerated, effective therapy for children with relapsed ALL and prospective studies are warranted. Identification of risk factors for developing post-HSCT SOS and strategies to mitigate this risk are ongoing.
Conflict of interest statement
MMO’B, EAR, MLL, and SRR have received research funding from Pfizer Inc. for different studies through grant mechanisms. Pfizer did not provide any financial support for this retrospective review of their compassionate use program. The remaining authors declare that they have no conflict of interest.
Figures
References
-
- Sun Weili, Malvar Jemily, Sposto Richard, Verma Anupam, Wilkes Jennifer J., Dennis Robyn, Heym Kenneth, Laetsch Theodore W., Widener Melissa, Rheingold Susan R, Oesterheld Javier, Hijiya Nobuko, Sulis Maria Luisa, Huynh Van, Place Andrew E., Bittencourt Henrique, Hutchinson Raymond, Messinger Yoav, Chang Bill, Matloub Yousif, Ziegler David S., Gardner Rebecca, Cooper Todd, Ceppi Francesco, Hermiston Michelle, Dalla-Pozza Luciano, Schultz Kirk R., Gaynon Paul, Wayne Alan S., Whitlock James A. Outcome of children with multiply relapsed B-cell acute lymphoblastic leukemia: a therapeutic advances in childhood leukemia & lymphoma study. Leukemia. 2018;32(11):2316–2325. doi: 10.1038/s41375-018-0094-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

