The molecular details of cytokine signaling via the JAK/STAT pathway
- PMID: 30267440
- PMCID: PMC6237706
- DOI: 10.1002/pro.3519
The molecular details of cytokine signaling via the JAK/STAT pathway
Abstract
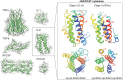
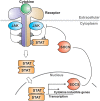
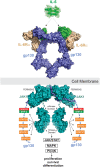
More than 50 cytokines signal via the JAK/STAT pathway to orchestrate hematopoiesis, induce inflammation and control the immune response. Cytokines are secreted glycoproteins that act as intercellular messengers, inducing proliferation, differentiation, growth, or apoptosis of their target cells. They act by binding to specific receptors on the surface of target cells and switching on a phosphotyrosine-based intracellular signaling cascade initiated by kinases then propagated and effected by SH2 domain-containing transcription factors. As cytokine signaling is proliferative and often inflammatory, it is tightly regulated in terms of both amplitude and duration. Here we review molecular details of the cytokine-induced signaling cascade and describe the architectures of the proteins involved, including the receptors, kinases, and transcription factors that initiate and propagate signaling and the regulatory proteins that control it.
Keywords: Cytokine Signaling; JAK/STAT; SOCS; cytokine; cytokine receptor; hematopoiesis.
© 2018 The Protein Society.
Figures





References
-
- Velazquez L, Fellous M, Stark GR, Pellegrini S (1992) A protein tyrosine kinase in the interferon‐alpha/beta signaling pathway. Cell 70:313–322. - PubMed
-
- Firmbach‐Kraft I, Byers M, Shows T, Dalla‐Favera R, Krolewski JJ (1990) tyk2, prototype of a novel class of non‐receptor tyrosine kinase genes. Oncogene 5:1329–1336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Operational Infrastructure Grant/Department of Health, State Government of Victoria/International
- 1113577/National Health and Medical Research Council/International
- 1121755/National Health and Medical Research Council/International
- 1122999/National Health and Medical Research Council/International
- 9000220/National Health and Medical Research Council/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

