Therapeutic strategies for enhancing angiogenesis in wound healing
- PMID: 30267742
- PMCID: PMC6435442
- DOI: 10.1016/j.addr.2018.09.010
Therapeutic strategies for enhancing angiogenesis in wound healing
Abstract
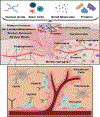
The enhancement of wound healing has been a goal of medical practitioners for thousands of years. The development of chronic, non-healing wounds is a persistent medical problem that drives patient morbidity and increases healthcare costs. A key aspect of many non-healing wounds is the reduced presence of vessel growth through the process of angiogenesis. This review surveys the creation of new treatments for healing cutaneous wounds through therapeutic angiogenesis. In particular, we discuss the challenges and advancement that have been made in delivering biologic, pharmaceutical and cell-based therapies as enhancers of wound vascularity and healing.
Keywords: Angiogenesis; Cell-based therapies; Chronic wounds; Diabetic ulcers; Growth factor therapy; Neovascularization; Non-healing wounds; Stem cell therapy; Wound healing.
Copyright © 2018. Published by Elsevier B.V.
Figures


References
-
- Snyder RJ, Kirsner RS, Warriner RA 3rd, Lavery LA, Hanft JR, Sheehan P, Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes, Ostomy/wound management, 56 (2010) S1–24. - PubMed
-
- Park NJ, Allen L, Driver VR, Updating on understanding and managing chronic wound, Dermatologic therapy, 26 (2013) 236–256. - PubMed
-
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT, Human skin wounds: a major and snowballing threat to public health and the economy, Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society, 17 (2009) 763–771. - PMC - PubMed
-
- Singer AJ, Clark RA, Cutaneous wound healing, The New England journal of medicine, 341 (1999) 738–746. - PubMed
-
- Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, Moroni M, Carabelli A, Platelet gel for healing cutaneous chronic wounds, Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis, 30 (2004) 145–151. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

