Vimentin and Non-Muscle Myosin IIA are Members of the Neural Precursor Cell Expressed Developmentally Down-Regulated 9 (NEDD9) Interactome in Head and Neck Squamous Cell Carcinoma Cells
- PMID: 30267961
- PMCID: PMC6160858
- DOI: 10.1016/j.tranon.2018.09.006
Vimentin and Non-Muscle Myosin IIA are Members of the Neural Precursor Cell Expressed Developmentally Down-Regulated 9 (NEDD9) Interactome in Head and Neck Squamous Cell Carcinoma Cells
Abstract
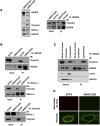
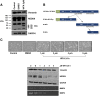
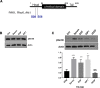
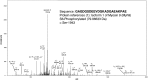
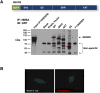
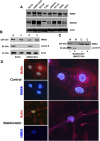
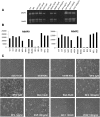
Here we demonstrate an interaction between neural precursor cell expressed, developmentally-downregulated 9 (NEDD9) and the cytoskeletal proteins vimentin and non-muscle myosin IIA (NMIIA), based on co-immunoprecipitation and mass spectrometric sequence identification. Vimentin was constitutively phosphorylated at Ser56 but vimentin associated with NEDD9-was not phosphorylated at Ser56. In contrast, NMIIA bound to NEDD9 was phosphorylated on S1943 consistent with its function in invasion and secretion. Treatment of cells with the vimentin-targeting steroidal lactone withaferin A had no effect on vimentin turnover as previously reported, instead causing NEDD9 cleavage and cell death. The NMIIA-selective inhibitor blebbistatin induced cells to form long extensions and attenuated secretion of matrix metalloproteinases (MMPs) 2 and 9. While the site of vimentin interaction on NEDD9 was not defined, NMIIA was found to interact with NEDD9 at its substrate domain. NEDD9 interactions with vimentin and NMIIA are consistent with these proteins having roles in MMP secretion and cell invasion. These findings suggest that a better understanding of NEDD9 signaling is likely to reveal novel therapeutic targets for the prevention of invasion and metastasis.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Withaferin-A induced vimentin S56 phosphorylation dissociates NEDD9 signaling loop to regress progressive metastatic melanoma into lung adenocarcinoma.Chem Biol Interact. 2025 Jan 25;406:111319. doi: 10.1016/j.cbi.2024.111319. Epub 2024 Nov 27. Chem Biol Interact. 2025. PMID: 39613173
-
NEDD9 stimulated MMP9 secretion is required for invadopodia formation in oral squamous cell carcinoma.Oncotarget. 2018 May 22;9(39):25503-25516. doi: 10.18632/oncotarget.25347. eCollection 2018 May 22. Oncotarget. 2018. PMID: 29876004 Free PMC article.
-
NEDD9 promotes invasion and migration of colorectal cancer cell line HCT116 via JNK/EMT.Oncol Lett. 2019 Oct;18(4):4022-4029. doi: 10.3892/ol.2019.10756. Epub 2019 Aug 16. Oncol Lett. 2019. PMID: 31516604 Free PMC article.
-
Roles of neural precursor cell expressed, developmentally downregulated 9 in tumor-associated cellular processes (Review).Mol Med Rep. 2015 Nov;12(5):6415-21. doi: 10.3892/mmr.2015.4240. Epub 2015 Aug 24. Mol Med Rep. 2015. PMID: 26324022 Review.
-
NEDD9 overexpression predicts poor prognosis in solid cancers: a meta-analysis.Onco Targets Ther. 2019 May 28;12:4213-4222. doi: 10.2147/OTT.S205760. eCollection 2019. Onco Targets Ther. 2019. PMID: 31213839 Free PMC article. Review.
Cited by
-
Non-Muscle Myosin II A: Friend or Foe in Cancer?Int J Mol Sci. 2024 Aug 30;25(17):9435. doi: 10.3390/ijms25179435. Int J Mol Sci. 2024. PMID: 39273383 Free PMC article. Review.
-
Comparative response to PDT with methyl-aminolevulinate and temoporfin in cutaneous and oral squamous cell carcinoma cells.Sci Rep. 2024 Mar 25;14(1):7025. doi: 10.1038/s41598-024-57624-8. Sci Rep. 2024. PMID: 38528037 Free PMC article.
-
MICAL-mediated oxidation of actin and its effects on cytoskeletal and cellular dynamics.Front Cell Dev Biol. 2023 Feb 17;11:1124202. doi: 10.3389/fcell.2023.1124202. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36875759 Free PMC article. Review.
-
Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review.Mol Cancer. 2019 Mar 30;18(1):63. doi: 10.1186/s12943-019-0983-5. Mol Cancer. 2019. PMID: 30927923 Free PMC article.
-
Investigating the Features of PDO Green Hams during Salting: Insights for New Markers and Genomic Regions in Commercial Hybrid Pigs.Animals (Basel). 2021 Jan 1;11(1):68. doi: 10.3390/ani11010068. Animals (Basel). 2021. PMID: 33401485 Free PMC article.
References
-
- Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125:1269–1281. - PubMed
-
- Loudig O, Brandwein-Gensler M, Kim RS, Lin J, Isayeva T, Liu C, Segall JE, Kenny PA, Prystowsky MB. Illumina whole-genome complementary DNA-mediated annealing, selection, extension and ligation platform: assessing its performance in formalin-fixed, paraffin-embedded samples and identifying invasion pattern-related genes in oral squamous cell carcinoma. Hum Pathol. 2011;42:1911–1922. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

