Cross-sectional increase of adherence to multidisciplinary tumor board decisions
- PMID: 30268109
- PMCID: PMC6162965
- DOI: 10.1186/s12885-018-4841-4
Cross-sectional increase of adherence to multidisciplinary tumor board decisions
Abstract
Background: Cancer research has made great progress in the recent years. With the increasing number of options in diagnosis and therapy the implementation of tumorboards (TUBs) has become standard procedure in the treatment of cancer patients. Adherence tests on tumor board decisions are intended to enable quality assurance and enhancement for work in tumor boards in order to continuously optimize treatment options for cancer patients.
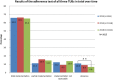
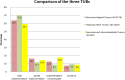
Methods: Subject of this study was the adherence of the recommendations made in three of 14 tumorboards, which take place weekly in the Center for Integrated Oncology (CIO) at the University Hospital Bonn. In total, therapy recommendations of 3815 patient cases were checked on their implementation. A classification into four groups has been made according to the degree of implementation. A second classification followed regarding the reasons for differences between the recommendation and the therapy which the patient actually received.
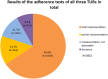
Results: The study showed that 80.1% of all recommendations in the three TUBs were implemented. 8.3% of all recommendations showed a deviance. Most important reasons for the deviances were patient wish (36.5%), patient death (26%) and doctoral decision, due to the patient's comorbidities or side effects of the treatment (24.1%).Interestingly, deviance in all three tumor boards in total significantly decreased over time.
Conclusions: Aim of the study was to clarify the use of tumor boards and find approaches to make them more efficient. Based on the results efficiency might be optimized by increased consideration of patients` preferences, improved presentation of patient-related data, more detailed documentation and further structuring of the tumor board meetings.
Keywords: Adherence; Deviance; Head and neck; Multidisciplinary meeting; Neurooncology; Sarcoma and musculoskeletal tumor; Tumor board.
Conflict of interest statement
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. This study was approved by our local ethics committee “Klinisches Ethikkommitee des UKB (KEK)”.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- McAvoy B. Optimising cancer care in Australia. Aust Fam Physician. 2003;32(5):369–372. - PubMed
-
- El Saghir N, Keating N, Carlson R, Khoury K, Fallowfield L. American Society of Clinical Oncology educational book American Society of Clinical Oncology meeting:e461-466. 2014. Tumor boards: optimizing the structure and improving efficiency of multidisciplinary management of patients with cancer worldwide. - PubMed
-
- Strohschneider T. Opening address by Prof. Dr. Siewert at the 23rd Congress of the German Cancer Society 8 June 1998 in Berlin "Oncology in the tension field between reality and vision". Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen. 70((6):suppl):156. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources