Peptide-Lipid Interaction Sites Affect Vesicles' Responses to Antimicrobial Peptides
- PMID: 30268538
- PMCID: PMC6372193
- DOI: 10.1016/j.bpj.2018.08.040
Peptide-Lipid Interaction Sites Affect Vesicles' Responses to Antimicrobial Peptides
Abstract
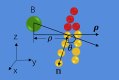
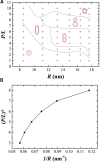
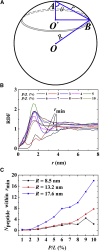
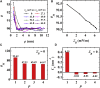
This article presents coarse-grained molecular dynamics simulations of pore-forming antimicrobial peptide melittin and its interactions with vesicles composed of a mixture of zwitterionic and anionic phospholipids. Besides creating holes in the membrane, the adsorption of melittin also induces vesicle budding, which can develop into vesiculation at high peptide concentrations, as well as vesicle invagination, which can eventually result in a corrugated membrane surface. These rich morphology changes are mediated by the curvature of the vesicles and the peptide concentration. Highly curved vesicles favor the recruitment of melittins with a higher density of binding sites. The peptides mainly penetrate into the membrane surface in monomers via hydrophobic interaction. Lowly curved vesicles recruit melittins with a low density of binding sites. Surplus peptides are prone to form oligomers and shallowly adsorb on the surface of membrane via electrostatic interaction. The penetration of monomers induces membrane pore formation and positive membrane curvature, which promote vesicle budding. The adsorption of oligomers induces negative membrane curvature, which promotes vesicle invagination. This work demonstrates that antimicrobial peptides adopt multiple actions to destroy bacterial membranes.
Copyright © 2018 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
A coarse-grained approach to studying the interactions of the antimicrobial peptides aurein 1.2 and maculatin 1.1 with POPG/POPE lipid mixtures.J Mol Model. 2018 Jul 17;24(8):208. doi: 10.1007/s00894-018-3747-z. J Mol Model. 2018. PMID: 30019106
-
Interaction of the Alzheimer Aβ(25-35) peptide segment with model membranes.Colloids Surf B Biointerfaces. 2016 May 1;141:10-18. doi: 10.1016/j.colsurfb.2016.01.015. Epub 2016 Jan 15. Colloids Surf B Biointerfaces. 2016. PMID: 26816349
-
Vesicle budding induced by a pore-forming peptide.J Am Chem Soc. 2010 Jan 13;132(1):195-201. doi: 10.1021/ja9059014. J Am Chem Soc. 2010. PMID: 20000420
-
Peptide:lipid ratio and membrane surface charge determine the mechanism of action of the antimicrobial peptide BP100. Conformational and functional studies.Biochim Biophys Acta. 2014 Jul;1838(7):1985-99. doi: 10.1016/j.bbamem.2014.04.004. Epub 2014 Apr 15. Biochim Biophys Acta. 2014. PMID: 24743023
-
Vesicle-Based Assays to Study Membrane Interactions of Amyloid Peptides.Methods Mol Biol. 2019;1873:39-51. doi: 10.1007/978-1-4939-8820-4_3. Methods Mol Biol. 2019. PMID: 30341602 Review.
Cited by
-
Antimicrobial Peptide Mastoparan-AF Kills Multi-Antibiotic Resistant Escherichia coli O157:H7 via Multiple Membrane Disruption Patterns and Likely by Adopting 3-11 Amphipathic Helices to Favor Membrane Interaction.Membranes (Basel). 2023 Feb 20;13(2):251. doi: 10.3390/membranes13020251. Membranes (Basel). 2023. PMID: 36837754 Free PMC article.
-
Membrane Remodeling by the Lytic Fragment of SticholysinII: Implications for the Toroidal Pore Model.Biophys J. 2019 Nov 5;117(9):1563-1576. doi: 10.1016/j.bpj.2019.09.018. Epub 2019 Sep 20. Biophys J. 2019. PMID: 31587828 Free PMC article.
-
How Alligator Immune Peptides Kill Gram-Negative Bacteria: A Lipid-Scrambling, Squeezing, and Extracting Mechanism Revealed by Theoretical Simulations.Int J Mol Sci. 2023 Jun 30;24(13):10962. doi: 10.3390/ijms241310962. Int J Mol Sci. 2023. PMID: 37446138 Free PMC article.
-
Structure and Formation Mechanism of Antimicrobial Peptides Temporin B- and L-Induced Tubular Membrane Protrusion.Int J Mol Sci. 2021 Oct 13;22(20):11015. doi: 10.3390/ijms222011015. Int J Mol Sci. 2021. PMID: 34681675 Free PMC article.
-
Polymyxin B Loosens Lipopolysaccharide Bilayer but Stiffens Phospholipid Bilayer.Biophys J. 2020 Jan 7;118(1):138-150. doi: 10.1016/j.bpj.2019.11.008. Epub 2019 Nov 16. Biophys J. 2020. PMID: 31812355 Free PMC article.
References
-
- Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. - PubMed
-
- Melo M.N., Ferre R., Castanho M.A. Antimicrobial peptides: linking partition, activity and high membrane-bound concentrations. Nat. Rev. Microbiol. 2009;7:245–250. - PubMed
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. - PubMed
-
- Powers J.P., Hancock R.E. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–1691. - PubMed
-
- Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta. 1999;1462:1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

