Modified carbazoles destabilize microtubules and kill glioblastoma multiform cells
- PMID: 30268825
- PMCID: PMC6690746
- DOI: 10.1016/j.ejmech.2018.09.026
Modified carbazoles destabilize microtubules and kill glioblastoma multiform cells
Abstract
Small molecules that target microtubules (MTs) represent promising therapeutics to treat certain types of cancer, including glioblastoma multiform (GBM). We synthesized modified carbazoles and evaluated their antitumor activity in GBM cells in culture. Modified carbazoles with an ethyl moiety linked to the nitrogen of the carbazole and a carbonyl moiety linked to distinct biaromatic rings exhibited remarkably different killing activities in human GBM cell lines and patient-derived GBM cells, with IC50 values from 67 to >10,000 nM. Measures of the activity of modified carbazoles with tubulin and microtubules coupled to molecular docking studies show that these compounds bind to the colchicine site of tubulin in a unique low interaction space that inhibits tubulin assembly. The modified carbazoles reported here represent novel chemical tools to better understand how small molecules disrupt MT functions and kill devastating cancers such as GBM.
Keywords: Carbazole; Colchicine; Gliomas; Microtubules.
Copyright © 2018 Elsevier Masson SAS. All rights reserved.
Figures
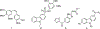

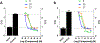
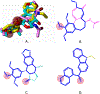
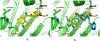
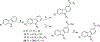
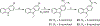

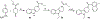
References
-
- Liu YM, Chen HL, Lee HY, Liou JP, Tubulin inhibitors: a patent review, Expert Opin Ther Pat, 24 (2014) 69–88. - PubMed
-
- Yoshida D, Hoshino S, Shimura T, Takahashi H, Teramoto A, Drug-induced apoptosis by anti-microtubule agent, estramustine phosphate on human malignant glioma cell line, U87MG; in vitro study, Journal of neuro-oncology, 47 (2000) 133–140. - PubMed
-
- Landen JW, Hau V, Wang M, Davis T, Ciliax B, Wainer BH, Van Meir EG, Glass JD, Joshi HC, Archer DR, Noscapine crosses the blood-brain barrier and inhibits glioblastoma growth, Clinical Cancer Research, 10 (2004) 5187–5201. - PubMed
-
- Boumendjel A, McLeer-Florin A, Champelovier P, Allegro D, Muhammad D, Souard F, Derouazi M, Peyrot V, Toussaint B, Boutonnat J, A novel chalcone derivative which acts as a microtubule depolymerising agent and an inhibitor of P-gp and BCRP in in-vitro and in-vivo glioblastoma models, BMC cancer, 9 (2009) 242. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

