Restored replication fork stabilization, a mechanism of PARP inhibitor resistance, can be overcome by cell cycle checkpoint inhibition
- PMID: 30269007
- PMCID: PMC7429716
- DOI: 10.1016/j.ctrv.2018.09.003
Restored replication fork stabilization, a mechanism of PARP inhibitor resistance, can be overcome by cell cycle checkpoint inhibition
Abstract
Poly(ADP-ribose) polymerase (PARP) inhibition serves as a potent therapeutic option eliciting synthetic lethality in cancers harboring homologous recombination (HR) repair defects, such as BRCA mutations. However, the development of resistance to PARP inhibitors (PARPis) poses a clinical challenge. Restoration of HR competency is one of the many molecular factors contributing to PARPi resistance. Combination therapy with cell cycle checkpoint (ATR, CHK1, and WEE1) inhibitors is being investigated clinically in many cancers, particularly in ovarian cancer, to enhance the efficacy and circumvent resistance to PARPis. Ideally, inhibition of ATR, CHK1 and WEE1 proteins will abrogate G2 arrest and subsequent DNA repair via restored HR in PARPi-treated cells. Replication fork stabilization has recently been identified as a potential compensatory PARPi resistance mechanism, found in the absence of restored HR. ATR, CHK1, and WEE1 each possess different roles in replication fork stabilization, providing different mechanisms to consider when developing combination therapies to avoid continued development of drug resistance. This review examines the impact of ATR, CHK1, and WEE1 on replication fork stabilization. We also address the therapeutic potential for combining PARPis with cell cycle inhibitors and the possible consequence of combination therapies which do not adequately address both restored HR and replication fork stabilization as PARPi resistance mechanisms.
Keywords: Cell cycle checkpoint inhibitors; Drug resistance; PARP inhibitor resistance; Replication fork protection; Replication fork stabilization.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no potential conflicts of interest.
Figures

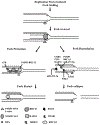
 ). Stalled forks can then be reversed by SMARCAL1 and these reversed forks can undergo fork protection by replication protein A (RPA; recruited by ATR), BRCA2, and/or RAD51 (recruited by PARP1 and BRCA2) and be restarted by RECQ1. Alternatively, these reversed forks can be degraded by EXO1, MRE11 (recruited by PTIP, CHD4, RAD52), and/or MUS81 (recruited by EZH2) mediated fork degradation and subsequent fork collapse and cell death. Notably, PARP1 and BRCA2 inhibits MRE11 mediated fork degradation and miR-493–5p blocks both MRE11 and EXO1 activity, supporting the role of PARP1, BRCA2, and miR-493–5p in fork protection and PARP inhibitor resistance.
). Stalled forks can then be reversed by SMARCAL1 and these reversed forks can undergo fork protection by replication protein A (RPA; recruited by ATR), BRCA2, and/or RAD51 (recruited by PARP1 and BRCA2) and be restarted by RECQ1. Alternatively, these reversed forks can be degraded by EXO1, MRE11 (recruited by PTIP, CHD4, RAD52), and/or MUS81 (recruited by EZH2) mediated fork degradation and subsequent fork collapse and cell death. Notably, PARP1 and BRCA2 inhibits MRE11 mediated fork degradation and miR-493–5p blocks both MRE11 and EXO1 activity, supporting the role of PARP1, BRCA2, and miR-493–5p in fork protection and PARP inhibitor resistance.
 :PARP-DNA lesion
:PARP-DNA lesion  :RPA.
:RPA.Similar articles
-
Cell cycle checkpoints and beyond: Exploiting the ATR/CHK1/WEE1 pathway for the treatment of PARP inhibitor-resistant cancer.Pharmacol Res. 2022 Apr;178:106162. doi: 10.1016/j.phrs.2022.106162. Epub 2022 Mar 5. Pharmacol Res. 2022. PMID: 35259479 Free PMC article. Review.
-
ATR, CHK1 and WEE1 inhibitors cause homologous recombination repair deficiency to induce synthetic lethality with PARP inhibitors.Br J Cancer. 2024 Sep;131(5):905-917. doi: 10.1038/s41416-024-02745-0. Epub 2024 Jul 4. Br J Cancer. 2024. PMID: 38965423 Free PMC article.
-
PARP Inhibition Increases the Reliance on ATR/CHK1 Checkpoint Signaling Leading to Synthetic Lethality-An Alternative Treatment Strategy for Epithelial Ovarian Cancer Cells Independent from HR Effectiveness.Int J Mol Sci. 2020 Dec 19;21(24):9715. doi: 10.3390/ijms21249715. Int J Mol Sci. 2020. PMID: 33352723 Free PMC article.
-
ATR inhibition disrupts rewired homologous recombination and fork protection pathways in PARP inhibitor-resistant BRCA-deficient cancer cells.Genes Dev. 2017 Feb 1;31(3):318-332. doi: 10.1101/gad.290957.116. Epub 2017 Feb 27. Genes Dev. 2017. PMID: 28242626 Free PMC article.
-
Exploiting replicative stress in gynecological cancers as a therapeutic strategy.Int J Gynecol Cancer. 2020 Aug;30(8):1224-1238. doi: 10.1136/ijgc-2020-001277. Epub 2020 Jun 22. Int J Gynecol Cancer. 2020. PMID: 32571890 Free PMC article. Review.
Cited by
-
DNA folds threaten genetic stability and can be leveraged for chemotherapy.RSC Chem Biol. 2020 Sep 30;2(1):47-76. doi: 10.1039/d0cb00151a. eCollection 2021 Feb 1. RSC Chem Biol. 2020. PMID: 35340894 Free PMC article. Review.
-
MCM10 is a Prognostic Biomarker and Correlated With Immune Checkpoints in Ovarian Cancer.Front Genet. 2022 May 19;13:864578. doi: 10.3389/fgene.2022.864578. eCollection 2022. Front Genet. 2022. PMID: 35664337 Free PMC article.
-
PARP Inhibitors Display Differential Efficacy in Models of BRCA Mutant High-Grade Serous Ovarian Cancer.Int J Mol Sci. 2021 Aug 7;22(16):8506. doi: 10.3390/ijms22168506. Int J Mol Sci. 2021. PMID: 34445211 Free PMC article.
-
Targeting the replication stress response through synthetic lethal strategies in cancer medicine.Trends Cancer. 2021 Oct;7(10):930-957. doi: 10.1016/j.trecan.2021.06.002. Epub 2021 Jun 30. Trends Cancer. 2021. PMID: 34215565 Free PMC article. Review.
-
A phase IA dose-escalation study of PHI-101, a new checkpoint kinase 2 inhibitor, for platinum-resistant recurrent ovarian cancer.BMC Cancer. 2022 Jan 3;22(1):28. doi: 10.1186/s12885-021-09138-z. BMC Cancer. 2022. PMID: 34980026 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

