Signs and symptoms of acromegaly at diagnosis: the physician's and the patient's perspectives in the ACRO-POLIS study
- PMID: 30269264
- PMCID: PMC6329724
- DOI: 10.1007/s12020-018-1764-4
Signs and symptoms of acromegaly at diagnosis: the physician's and the patient's perspectives in the ACRO-POLIS study
Erratum in
-
Correction to: Signs and symptoms of acromegaly at diagnosis: the physician's and the patient's perspectives in the ACRO-POLIS study.Endocrine. 2019 Jan;63(1):130. doi: 10.1007/s12020-018-1789-8. Endocrine. 2019. PMID: 30382552 Free PMC article.
Abstract
Purpose: Acromegaly is characterized by a broad range of manifestations. Early diagnosis is key to treatment success, but is often delayed as symptomatology overlaps with common disorders. We investigated sign-and-symptom associations, demographics, and clinical characteristics at acromegaly diagnosis.
Methods: Observational, cross-sectional, multicenter non-interventional study conducted at 25 hospital departments in France that treat acromegaly (ClinicalTrials.gov: NCT02012127). Adults diagnosed with acromegaly < 5 years were enrolled. Demographic and clinical data were obtained from medical reports and patient questionnaires. Sign-and-symptom associations were assessed by multiple correspondence analysis (MCA).
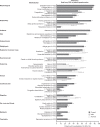
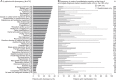
Results: Overall, 472 patients were included in the analyses. MCA was unsuccessful in identifying sign-and-symptom associations at diagnosis. Endocrinologists (29.5% patients) and other clinical specialists (37.2% patients) were commonly first to suspect acromegaly. Morphologic manifestations (83.7-87.9% patients), snoring syndrome (81.4% patients), and asthenia (79.2% patients) were frequently present at diagnosis; differences were found between sexes for specific manifestations. Rates of discrepancy between patient- and physician-reported manifestations were highest for functional signs. Earliest manifestations prior to diagnosis, according to how they were detected, were enlarged hands and feet (6.4 ± 6.8 and 6.2 ± 6.9 years, functional signs), hypertension (6.6 ± 7.5 years, complementary examination) and carpal/cubital tunnel syndrome (5.7 ± 6.7 years, functional signs with complementary examination).
Conclusions: Results confirm the broad range of manifestations at diagnosis and delay in recognizing the disease. We identified early manifestations and sex differences that may aid physicians in diagnosing acromegaly. Discrepancy rates suggest physicians should obtain the patient's perspective and seek functional signs during diagnosis.
Keywords: Acromegaly; Diagnosis; Multiple correspondence analysis; Sign-and-symptom association.
Conflict of interest statement
P.C. is a consultant and speaker for Ipsen, Novartis and Pfizer, and an advisory board member for Ipsen. T.B. has received institutional research support from Novo-Nordisk, Sandoz and Pfizer, consultancy or lectureship fees from Ipsen, Novartis, Strongbridge, and Pfizer, and has served as investigator for clinical trials funded by Novartis, Ipsen and Strongbridge. G.R. has received research grants from Ipsen and Novartis; has served as investigator (principal or coordinator) for clinical trials funded by Novartis and Ipsen, and is a member of Advisory Boards for Ipsen and Novartis. A.T. is a consultant for Ipsen, Novartis, and HRA Pharma and has received lecture fees from Novartis, HRA Pharma and Pfizer. A.C. has no financial disclosures. B.D. has received unrestricted research grants from Pfizer and Novartis, and has served as investigator for clinical trials funded by Novartis, Ipsen and Pfizer. B.D. is a member of advisory board for Ipsen, Novartis and Pfizer and has given lectures for Ipsen, Novartis and Pfizer. PPR has served as coinvestigator for clinical trials funded by Pfizer and Ipsen. A.H. is an employee of Ipsen. F.E. is an employee of Ipsen. P.C. has received unrestricted research and educational grants from Ipsen, Novartis, and Pfizer as Head of the Department of Endocrinology and Reproductive Diseases, Hôpitaux Universitaires Paris-Sud. P.C. has served as investigator (principal or coordinator) for clinical trials funded by Novartis, Pfizer, Ipsen, Italfarmaco, and Antisense Therapeutics. P.C. is a member of Advisory Boards for Ipsen and Novartis. P.C. has given lectures for Ipsen, Novartis, and Pfizer. All fees and honoraria are paid to his Institution.
Figures


References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

