Therapeutic regimen of L-arginine for MELAS: 9-year, prospective, multicenter, clinical research
- PMID: 30269300
- PMCID: PMC6244654
- DOI: 10.1007/s00415-018-9057-7
Therapeutic regimen of L-arginine for MELAS: 9-year, prospective, multicenter, clinical research
Abstract
Objective: To examine the efficacy and safety of the therapeutic regimen using oral and intravenous L-arginine for pediatric and adult patients with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS).
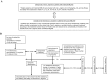
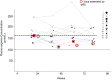
Methods: In the presence and absence of an ictus of stroke-like episodes within 6 h prior to efficacy assessment, we correspondingly conducted the systematic administration of oral and intravenous L-arginine to 15 and 10 patients with MELAS in two, 2-year, prospective, multicenter clinical trials at 10 medical institutions in Japan. Subsequently, patients were followed up for 7 years. The primary endpoint in the clinical trial of oral L-arginine was the MELAS scale, while that for intravenous L-arginine was the improvement rates of headache and nausea/vomiting at 2 h after completion of the initial intravenous administration. The relationships between the ictuses of stroke-like episodes and plasma arginine concentrations were examined.
Results: Oral L-arginine extended the interictal phase (p = 0.0625) and decreased the incidence and severity of ictuses. Intravenous L-arginine improved the rates of four major symptoms-headache, nausea/vomiting, impaired consciousness, and visual disturbance. The maximal plasma arginine concentration was 167 μmol/L when an ictus developed. Neither death nor bedriddenness occurred during the 2-year clinical trials, and the latter did not develop during the 7-year follow-up despite the progressively neurodegenerative and eventually life-threatening nature of MELAS. No treatment-related adverse events occurred, and the formulations of L-arginine were well tolerated.
Conclusions: The systematic administration of oral and intravenous L-arginine may be therapeutically beneficial and clinically useful for patients with MELAS.
Keywords: Ictus; L-Arginine; MELAS; Mitochondrial disease; Stroke-like episodes.
Conflict of interest statement
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical standards
The research was performed at 10 medical institutions in Japan in accordance with international guidelines, including the Declaration of Helsinki, Good Clinical Practice, and Council for International Organizations of Medical Sciences, International Ethical Guidelines (NCT02367014). The study protocol was approved by individual institutional review committees.
Informed consent
Written informed consent was obtained from all subjects or from his or her legal representative prior to enrolment.
Figures

Comment in
-
Whether NO-precursors are truly beneficial for stroke-like episodes remains unsolved.J Neurol. 2019 Jan;266(1):245-246. doi: 10.1007/s00415-018-9090-6. Epub 2018 Oct 15. J Neurol. 2019. PMID: 30324306 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

