Dimeric FcγR ectodomains detect pathogenic anti-platelet factor 4-heparin antibodies in heparin-induced thromobocytopenia
- PMID: 30269432
- PMCID: PMC6635755
- DOI: 10.1111/jth.14306
Dimeric FcγR ectodomains detect pathogenic anti-platelet factor 4-heparin antibodies in heparin-induced thromobocytopenia
Abstract
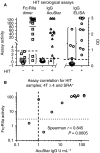
Essentials FcγRIIa mediates life-threatening heparin-induced thrombocytopenia (HIT). Most anti-platelet factor (PF)4-heparin IgGs are not pathogenic so diagnosis of HIT is challenging. Dimeric rsFcγRIIa was used to quantify receptor-binding activity of anti-PF4-heparin antibodies. Dimeric rsFcγRIIa binding specifically correlated with occurrence of HIT. SUMMARY: Background Heparin-induced thrombocytopenia (HIT) is a major and potentially fatal consequence of antibodies produced against platelet factor 4 (PF4)-heparin complexes following heparin exposure. Not all anti-PF4-heparin antibodies are pathogenic, so overdiagnosis can occur, with resulting inappropriate use of alternative anticoagulation therapies that have associated risks of bleeding. However, definitive platelet functional assays are not widely available for routine analysis. Objectives To assess the utility of dimeric recombinant soluble FcγRIIa (rsFcγRIIa) ectodomains for detecting HIT antibodies. Patients/Methods Plasma from 27 suspected HIT patients were tested for pathogenic anti-PF4-heparin antibodies by binding of a novel dimeric FcγRIIa ectodomain probe. Plasmas were also tested by the use of PF4-heparin IgG ELISA, the HemosIL AcuStar HIT IgG-specific assay, and a serotonin release assay (SRA). Results The dimeric rsFcγRIIa test produced no false positives and excluded four samples that were positive by IgG ELISA. In this small patient cohort, the novel assay correctly assigned 93% of the suspected HIT patients, with two of the HIT patients being scored as false negatives. The improved discrimination of the novel assay over the IgG ELISA, which scored four false positives, supports the mechanistic interpretation that binding of dimeric rsFcγRIIa detects pairs of closely spaced IgG antibodies in PF4-heparin immune complexes. Conclusions This study found the cell-free, function-based dimeric rsFcγRIIa assay to be convenient, simple, and potentially predictive of HIT. The assay had improved specificity over the IgG ELISA, and correlated strongly with the AcuStar HIT IgG-specific assay, warranting further evaluation of its potential to identify HIT in larger patient cohorts.
Keywords: enzyme immunoassay; heparin; platelet factor 4; thrombocytopenia; thrombosis.
©2018 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals, Inc. on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
P. M. Hogarth and B. D. Wines report receiving grants from the Australian Centre for HIV and Hepatitis Virology Research during the conduct of the study, and have a patent issued (to the Burnet Institute): ‘Binding assays and method for probing antibody function with fc binding multimers WO 2017054033 A1’. S. Esparon reports receiving grants from the Australian Centre for HIV and Hepatitis Virology Research during the conduct of the study. R. Baker reports providing clinical trial support for Biogen Idec, Boehringer Ingelheim, Bayer, Shire, Pfizer, Daiichi Sankyo, Portola, Alexion Pharmaceuticals, Astellas, and CSL Behring, serving on clinical advisory boards of Bayer, Shire, Pfizer, and Amgen, and receiving research support from Bristol‐Meyers Squibb, Shire, and Bayer. E. Duncan reports receiving personal fees from Novo Nordisk. The other authors state that they have no conflict of interest.
Figures

Similar articles
-
Evaluation of a new automated panel of assays for the detection of anti-PF4/heparin antibodies in patients suspected of having heparin-induced thrombocytopenia.Thromb Haemost. 2010 Aug;104(2):402-9. doi: 10.1160/TH10-01-0002. Epub 2010 Jun 10. Thromb Haemost. 2010. PMID: 20539902
-
Beneficial effect of exogenous platelet factor 4 for detecting pathogenic heparin-induced thrombocytopenia antibodies.Br J Haematol. 2017 Dec;179(5):811-819. doi: 10.1111/bjh.14955. Epub 2017 Oct 19. Br J Haematol. 2017. PMID: 29048130
-
Distinguishing between anti-platelet factor 4/heparin antibodies that can and cannot cause heparin-induced thrombocytopenia.J Thromb Haemost. 2015 Oct;13(10):1900-7. doi: 10.1111/jth.13066. Epub 2015 Aug 29. J Thromb Haemost. 2015. PMID: 26291604
-
An update on the prevalence and characterization of H-PF4 antibodies in Asian-Indian patients.Semin Thromb Hemost. 2009 Apr;35(3):337-43. doi: 10.1055/s-0029-1222612. Epub 2009 May 18. Semin Thromb Hemost. 2009. PMID: 19452409 Review.
-
Testing for heparin-induced thrombocytopenia antibodies.Transfus Med Rev. 2006 Oct;20(4):259-72. doi: 10.1016/j.tmrv.2006.05.001. Transfus Med Rev. 2006. PMID: 17008164 Review.
Cited by
-
The Human FcγRII (CD32) Family of Leukocyte FcR in Health and Disease.Front Immunol. 2019 Mar 19;10:464. doi: 10.3389/fimmu.2019.00464. eCollection 2019. Front Immunol. 2019. PMID: 30941127 Free PMC article. Review.
-
Fc Binding by FcγRIIa Is Essential for Cellular Activation by the Anti-FcγRIIa mAbs 8.26 and 8.2.Front Immunol. 2021 Oct 25;12:666813. doi: 10.3389/fimmu.2021.666813. eCollection 2021. Front Immunol. 2021. PMID: 34759915 Free PMC article.
-
Concern About the Adverse Effects of Thrombocytopenia and Thrombosis After Adenovirus-Vectored COVID-19 Vaccination.Clin Appl Thromb Hemost. 2021 Jan-Dec;27:10760296211040110. doi: 10.1177/10760296211040110. Clin Appl Thromb Hemost. 2021. PMID: 34541935 Free PMC article. Review.
References
-
- Fabris F, Luzzatto G, Stefani PM, Girolami B, Cella G, Girolami A. Heparin‐induced thrombocytopenia. Haematologica 2000; 85: 72–81. - PubMed
-
- Greinacher A. Heparin‐induced thrombocytopenia. N Engl J Med 2015; 373: 1883–4. - PubMed
-
- Qiao J, Al‐Tamimi M, Baker RI, Andrews RK, Gardiner EE. The platelet Fc receptor, FcγRIIa. Immunol Rev 2015; 268: 241–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

