Recall by genotype and cascade screening for familial hypercholesterolemia in a population-based biobank from Estonia
- PMID: 30270359
- PMCID: PMC6443485
- DOI: 10.1038/s41436-018-0311-2
Recall by genotype and cascade screening for familial hypercholesterolemia in a population-based biobank from Estonia
Abstract
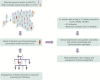
Purpose: Large-scale, population-based biobanks integrating health records and genomic profiles may provide a platform to identify individuals with disease-predisposing genetic variants. Here, we recall probands carrying familial hypercholesterolemia (FH)-associated variants, perform cascade screening of family members, and describe health outcomes affected by such a strategy.
Methods: The Estonian Biobank of Estonian Genome Center, University of Tartu, comprises 52,274 individuals. Among 4776 participants with exome or genome sequences, we identified 27 individuals who carried FH-associated variants in the LDLR, APOB, or PCSK9 genes. Cascade screening of 64 family members identified an additional 20 carriers of FH-associated variants.
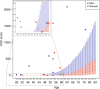
Results: Via genetic counseling and clinical management of carriers, we were able to reclassify 51% of the study participants from having previously established nonspecific hypercholesterolemia to having FH and identify 32% who were completely unaware of harboring a high-risk disease-associated genetic variant. Imaging-based risk stratification targeted 86% of the variant carriers for statin treatment recommendations.
Conclusion: Genotype-guided recall of probands and subsequent cascade screening for familial hypercholesterolemia is feasible within a population-based biobank and may facilitate more appropriate clinical management.
Keywords: cascade screening; familial hypercholesterolemia; genomics-guided disease management; population-based biobank; recall by genotype.
Conflict of interest statement
A.P. is a Venture Partner at GV, which is part of Alphabet Corporation; in that capacity he receives monetary compensation. S.K. has received grants and personal fees from Bayer and Amarin Pharma, Inc.; compensation as a member of the scientific advisory board from Catabasis, Regeneron Genetics Center, Merck, Celera, GENOMICS plc, Corvidia Therapeutics, Novo Ventures; and is affiliated with and received compensation from San Therapeutics, Novartis, AstraZeneca, Alynlam, Eli Lilly, Leerink Partners, Noble Insights, Ionis, Haug Partners LLC, Genetic Modifiers Newco Inc., Morgan Stanley Institutional Equity Division, ExpertConnect. Additionally, S.K. has a patent number WO2016086197 “Method of identifying and treating a person having predisposition to or afflicted with a cardiometabolic disease” issued. The other authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

