Effects of meal replacement therapy on metabolic outcomes in Thai patients with type 2 diabetes: A randomized controlled trial
- PMID: 30270717
- PMCID: PMC6340108
- DOI: 10.1177/0260106018800074
Effects of meal replacement therapy on metabolic outcomes in Thai patients with type 2 diabetes: A randomized controlled trial
Abstract
Background: A meal replacement (MR) with a low glycemic index (GI) is possibly beneficial for glycemic control. However, the effects of MR on diabetes mellitus have not been studied among Thai patients with type 2 diabetes (T2DM).
Aim: To compare metabolic outcomes between T2DM patients receiving the new MR formula (ONCE PRO) and normal controlled diets.
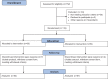
Methods: A multicenter, open-labeled, randomized controlled trial was conducted. Eligible patients received either ONCE PRO for one meal daily with controlled diets or only controlled diets for 3 months. The differences in metabolic profile between the baseline and end point of each group and between groups were measured.
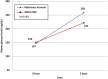
Results: 110 participants were enrolled; the mean difference and standard deviation in hemoglobin A1C (HbA1c) (%) from baseline were -0.21 ± 0.78 (p = 0.060) and -0.27 ± 0.60 (p = 0.001) in the MR and control groups, respectively; however, there was no significant difference between groups (p = 0.637). Patients consuming a MR instead of breakfast had a significant decrease in HbA1c (p = 0.040). Body weight (BW) and body mass index (BMI) were significantly reduced in both groups. There were no significant change in waist circumference, fasting plasma glucose, total cholesterol and triglycerides. Low-density lipoprotein cholesterol (LDL-C) was significantly decreased in the MR group compared with the control group (p = 0.049).
Conclusions: Short-term conventional diet control and the low-GI MR product were associated with a decreased BW and BMI. Changes in the other metabolic outcomes, HbA1c, total cholesterol and triglycerides, were comparable despite ONCE PRO as the MR having a better effect on LDL-C lowering.
Keywords: ONCE PRO; glycemic control; meal replacement; randomized controlled trial; type 2 diabetes.
Conflict of interest statement
Figures
Similar articles
-
Individualized Meal Replacement Therapy Improves Clinically Relevant Long-Term Glycemic Control in Poorly Controlled Type 2 Diabetes Patients.Nutrients. 2018 Aug 4;10(8):1022. doi: 10.3390/nu10081022. Nutrients. 2018. PMID: 30081574 Free PMC article. Clinical Trial.
-
Taking a low glycemic index multi-nutrient supplement as breakfast improves glycemic control in patients with type 2 diabetes mellitus: a randomized controlled trial.Nutrients. 2014 Dec 10;6(12):5740-55. doi: 10.3390/nu6125740. Nutrients. 2014. PMID: 25514391 Free PMC article. Clinical Trial.
-
Meal replacement reduces insulin requirement, HbA1c and weight long-term in type 2 diabetes patients with >100 U insulin per day.J Hum Nutr Diet. 2014 Apr;27 Suppl 2:21-7. doi: 10.1111/jhn.12145. Epub 2013 Aug 2. J Hum Nutr Diet. 2014. PMID: 23909831
-
Impact of High-Carbohydrate Diet on Metabolic Parameters in Patients with Type 2 Diabetes.Nutrients. 2017 Mar 24;9(4):322. doi: 10.3390/nu9040322. Nutrients. 2017. PMID: 28338608 Free PMC article. Review.
-
Low-glycemic index diets as an intervention for diabetes: a systematic review and meta-analysis.Am J Clin Nutr. 2019 Oct 1;110(4):891-902. doi: 10.1093/ajcn/nqz149. Am J Clin Nutr. 2019. PMID: 31374573
Cited by
-
Diabetes-specific formula with standard of care improves glycemic control, body composition, and cardiometabolic risk factors in overweight and obese adults with type 2 diabetes: results from a randomized controlled trial.Front Nutr. 2024 Jul 15;11:1400580. doi: 10.3389/fnut.2024.1400580. eCollection 2024. Front Nutr. 2024. PMID: 39077157 Free PMC article.
-
The Health Effects of Low Glycemic Index and Low Glycemic Load Interventions on Prediabetes and Type 2 Diabetes Mellitus: A Literature Review of RCTs.Nutrients. 2023 Dec 10;15(24):5060. doi: 10.3390/nu15245060. Nutrients. 2023. PMID: 38140319 Free PMC article. Review.
-
Meal replacements on obesity and leptin: a systematic review and meta-analysis.Rev Endocr Metab Disord. 2025 Feb;26(1):55-80. doi: 10.1007/s11154-024-09918-5. Epub 2024 Oct 21. Rev Endocr Metab Disord. 2025. PMID: 39433654
-
Impact of Partial Meal Replacement on Glycemic Levels and Body Weight in Indian Patients with Type 2 Diabetes (PRIDE): A Randomized Controlled Study.Diabetes Ther. 2022 Sep;13(9):1599-1619. doi: 10.1007/s13300-022-01294-0. Epub 2022 Jul 14. Diabetes Ther. 2022. PMID: 35834107 Free PMC article.
-
Efficacy and Sustainability of Diabetes-Specific Meal Replacement on Obese and Overweight Type-2 Diabetes Mellitus Patients: Study Approaches for a Randomised Controlled Trial and Impact of COVID-19 on Trial Progress.Int J Environ Res Public Health. 2022 Apr 1;19(7):4188. doi: 10.3390/ijerph19074188. Int J Environ Res Public Health. 2022. PMID: 35409872 Free PMC article. Clinical Trial.
References
-
- Abbott (2014) Glucerna® 1.2 CAL. Available at: https://abbottnutrition.com/glucerna-1_2-cal (accessed 23 September).
-
- Allison DB, Gadbury G, Schwartz LG, et al. (2003) A novel soy-based meal replacement formula for weight loss among obese individuals: a randomized controlled clinical trial. European Journal of Clinical Nutrition 57: 514. - PubMed
-
- American Diabetes Association (2014) Standards of medical care in diabetes—2014. Diabetes care 37(S1): S14–S80. - PubMed
-
- American Diabetes Association (2015) 4. Foundations of care: education, nutrition, physical activity, smoking cessation, psychosocial care, and immunization. Diabetes care 38(S1): S20–S30. - PubMed
-
- Aston LM. (2006) Glycaemic index and metabolic disease risk. Proceedings of the Nutrition Society 65: 125–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical