Behavioral and Neural Subsystems of Rodent Exploration
- PMID: 30270939
- PMCID: PMC6159932
- DOI: 10.1016/j.lmot.2017.03.009
Behavioral and Neural Subsystems of Rodent Exploration
Abstract
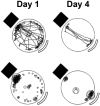
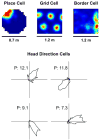
Animals occupy territories in which resources such as food and shelter are often distributed unevenly. While studies of exploratory behavior have typically involved the laboratory rodent as an experimental subject, questions regarding what constitutes exploration have dominated. A recent line of research has utilized a descriptive approach to the study of rodent exploration, which has revealed that this behavior is organized into movement subsystems that can be readily quantified. The movements include home base behavior, which serves as a central point of attraction from which rats and mice organize exploratory trips into the remaining environment. In this review, we describe some of the features of this organized behavior pattern as well as its modulation by sensory cues and previous experience. We conclude the review by summarizing research investigating the neurobiological bases of exploration, which we hope will stimulate renewed interest and research on the neural systems mediating rodent exploratory behavior.
Keywords: grid cells; head direction cells; hippocampus; hyperactivity; locomotor; navigation; open-field; place cell; spatial behavior.
Figures



References
-
- Alyan SH. Evidence for resetting the directional component of path integration in the house mouse. Ethology. 1996;102:629–638.
-
- Avni R, Elkan T, Dror AA, Shefer S, Eilam D, Avraham KB, Mintz M. Mice with vestibular deficiency display hyperactivity, disorientation, and signs of anxiety. Behav Brain Res. 2009;202:210–7. - PubMed
-
- Barnett SA. The Rat: A Study of Behavior. Aldine, Chicago: 1963.
-
- Barry C, Hayman R, Burgess N, Jeffery KJ. Experience-dependent rescaling of entorhinal grids. Nat Neurosci. 2007;10:682–4. - PubMed
-
- Berlyne DE. Conflict, arousal, and curiosity. New York, NY, US: McGraw-Hill Book Company; 1960.
Grants and funding
LinkOut - more resources
Full Text Sources
