Emerging Therapies in the Management of Advanced-Stage Gastric Cancer
- PMID: 30271341
- PMCID: PMC6146175
- DOI: 10.3389/fphar.2018.00404
Emerging Therapies in the Management of Advanced-Stage Gastric Cancer
Abstract
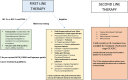
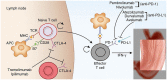
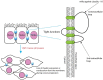
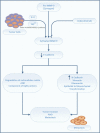
Globally, gastric malignancy contributes to significant cancer-related morbidity and mortality. Despite a recent approval of two targeted agents, trastuzumab and ramucirumab, the treatment options for advanced-stage gastric cancer are limited. Consequently, the overall clinical outcomes for patients with advanced-stage gastric cancer remain poor. Numerous agents that are active against novel targets have been evaluated in the course of randomized trials; however, most have produced disappointing results because of the molecular heterogeneity of gastric cancer. The Cancer Genome Atlas (TCGA) project proposed a new classification system for gastric cancer that includes four different tumor subtypes based on molecular characteristics. This change led to the identification of several distinct and potentially targetable pathways. However, most agents targeting these pathways do not elicit any meaningful clinical benefit when employed for the treatment of advanced-stage gastric cancer. Most advanced-stage gastric cancer trials currently focus on agents that modulate tumor microenvironments and cancer cell stemness. In this review, we summarize data regarding novel compounds that have shown efficacy in early phase studies and show promise as effective therapeutic agents, with special emphasis on those for which phase III trials are either planned or underway.
Keywords: The Cancer Genome Atlas; andecaliximab; claudiximab; gastric cancer; gastroesophageal cancer; immune checkpoint inhibitor; immunotherapy; napabucasin.
Figures
References
-
- Aggarwal B. B., Kunnumakkara A. B., Harikumar K. B., Gupta S. R., Tharakan S. T., Koca C., et al. . (2009). Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann. N. Y. Acad. Sci. 1171, 59–76. 10.1111/j.1749-6632.2009.04911.x - DOI - PMC - PubMed
-
- Bang Y.-J., Giaccone G., Im S., Oh D., Bauer T., Nordstrom J., et al. . (2017c). First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 28, 855–861. 10.1093/annonc/mdx002 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

