Mesenchymal Stromal Cells Based Therapy in Systemic Sclerosis: Rational and Challenges
- PMID: 30271402
- PMCID: PMC6146027
- DOI: 10.3389/fimmu.2018.02013
Mesenchymal Stromal Cells Based Therapy in Systemic Sclerosis: Rational and Challenges
Abstract
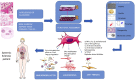
Systemic Sclerosis (SSc) is a rare chronic disease, related to autoimmune connective tissue diseases such as Systemic Lupus Erythematosus and Sjögren's Syndrome. Although its clinical heterogeneity, main features of the disease are: extensive tissue fibrosis with increase matrix deposition in skin and internal organ, microvascular alterations and activation of the immune system with autoantibodies against various cellular antigens. In the diffuse cutaneous scleroderma subtype, the disease is rapidly progressive with a poor prognosis, leading to failure of almost any internal organ, especially lung which is the leading cause of death. Primary trigger is unknown but may involve an immune process against mesenchymal cells in a genetically receptive host. Pathophysiology reveals a pivotal role of fibrosis and inflammation alterations implicating different cell subtypes, cytokines and growth factors, autoantibodies and reactive oxygen species. Despite improvement, the overall survival of SSc patients is still lower than that of other inflammatory diseases. Recommended drugs are agents capable of modulating fibrotic and inflammatory pathways. Cellular therapy has recently emerged as a credible option. Besides autologous hematopoietic stem cell transplantation which demonstrated remarkable improvement, mesenchymal stromal cells (MSCs) represent promising therapeutic candidates. Indeed, these cells possess anti-inflammatory, antiproliferative, antifibrotic, and immunomodulary properties especially by secreting a large panel of bioactive molecules, addressing the most important key points of the SSc. In addition, these cells are very sensitive to their environment and are able to modulate their activity according to the pathophysiological context in which they are located. Autologous or allogeneic MSCs from various sources have been tested in many trials in different auto-immune diseases such as multiple sclerosis, Crohn's disease or systemic lupus erythematosus. They are characterized by a broad availability and no or low acute toxicity. However, few randomized prospective clinical trials were published and their production under ATMP regulatory procedures is complex and time-consuming. Many aspects have still to be addressed to ascertain their potential as well as the potential of their derived products in the management of SSc, probably in association with other therapies.
Keywords: cell therapy; good manufacturing procedures; immunomodulation; mesenchymal stromal cells; systemic sclerosis.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

