MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction
- PMID: 30271437
- PMCID: PMC6151206
- DOI: 10.1155/2018/3290372
MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction
Abstract
Background: To cure ischemic diseases, angiogenesis needs to be improved by various strategies in ischemic area. Considering that microRNA-132 (miR-132) regulates endothelial cell behavior during angiogenesis and the safe and efficacious delivery of microRNAs in vivo is rarely achieved, an ideal vehicle for miR-132 delivery could bring the promise for ischemic diseases. As a natural carrier of biological molecules, exosomes are more and more developed as an ideal vehicle for miRNA transfer. Meanwhile, mesenchymal stem cells could release large amounts of exosomes. Thus, this study aimed to investigate whether MSC-derived exosomes can be used for miR-132 delivery in the treatment of myocardial ischemia.
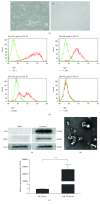
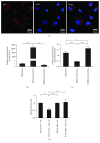
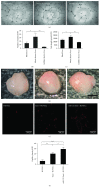
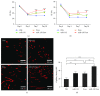
Methods: MSC-derived exosomes were electroporated with miR-132 mimics and inhibitors. After electroporation, miR-132 exosomes were labelled with DiI and added to HUVECs. Internalization of DiI-labelled exosomes was examined by fluorescent microscopy. Expression levels of miR-132 in exosomes and HUVECs were quantified by real-time PCR. The mRNA levels of miR-132 target gene RASA1 in HUVECs were quantified by real-time PCR. Luciferase reporter assay was performed to examine the targeting relationship between miR-132 and RASA1. The effects of miR-132 exosomes on the angiogenic ability of endothelial cells were evaluated by tube formation assay. Matrigel plug assay and myocardial infarction model were used to determine whether miR-132 exosomes can promote angiogenesis in vivo.
Results: miR-132 mimics were effectively electroporated and highly detected in MSC-derived exosomes. The expression level of miR-132 was high in HUVECs preincubated with miR-132 mimic-electroporated exosomes and low in HUVECs preincubated with miR-132 inhibitor-electroporated exosomes. The expression level of RASA1, miR-132 target gene, was reversely correlated with miR-132 expression in HUVECs pretreated with exosomes. Luciferase reporter assay further confirmed that RASA1 was a direct target of miR-132. Exosomes loaded with miR-132, as a vehicle for miRNA transfer, significantly increased tube formation of endothelial cells. Moreover, subcutaneous injection of HUVECs pretreated with miR-132 exosomes in nude mice significantly increased their angiogenesis capacity in vivo. In addition, transplantation of miR-132 exosomes in the ischemic hearts of mice markedly enhanced the neovascularization in the peri-infarct zone and preserved heart functions.
Conclusions: The findings suggest that the export of miR-132 via MSC-derived exosomes represents a novel strategy to enhance angiogenesis in ischemic diseases.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

