Improved Long-Term Clinical Outcomes And Safety Profile Of Sunitinib Dosing Schedule With 4/2 Switched To 2/1 In Patients With Metastatic Renal Cell Carcinoma
- PMID: 30271490
- PMCID: PMC6160671
- DOI: 10.7150/jca.25693
Improved Long-Term Clinical Outcomes And Safety Profile Of Sunitinib Dosing Schedule With 4/2 Switched To 2/1 In Patients With Metastatic Renal Cell Carcinoma
Abstract
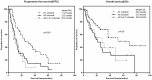
Purpose: This study aimed to identify the survival benefit and safety of alternative dosage schedules for sunitinib in metastatic renal cell carcinoma. Materials and Methods: Clinicopathologic and survival data of patients treated with sunitinib as first-line therapy were retrospectively reviewed. Patients were classified into three groups: a standard dosing schedule (4/2 schedule), alternative dosing schedule (2/1 schedule), and switched dosing schedule (4/2-2/1 schedule). Results: Ninety-nine patients were retrospectively included. Seventy-five (75.8%) patients were initially administrated with a 4/2 schedule of sunitinib, while 24 were started with the 2/1 schedule. During treatment, 45 (60.0%) patients with an initial 4/2 schedule switched to a 2/1 schedule (4/2-2/1 schedule) due to severe adverse events (AEs) or poor tolerance. Compared to that with a 4/2 schedule, patients with a 2/1 schedule had a much lower incidence of grade 3/4 AEs (69.6% vs. 40.6%, p=0.001). Overall, the 4/2-2/1 schedule was associated with the best survival benefits. Among the 4/2, 2/1, and 4/2-2/1 schedule groups, the median PFS was 12.5, 11.0, and 25.0 months, respectively (p=0.003), and the median OS was 21.0, 28.0, and 52.0 months, respectively (p=0.03). Multivariate analysis identified the 4/2-2/1 schedule as an independent factor predicting favorable PFS. Although without statistical significance, 4/2-2/1 schedule could decrease 55% risk of death. Furthermore, patients with unfavorable IMDC risk seemed to have more opportunity to achieve better survival from the 4/2-2/1 dosing schedule. Conclusion: Patients with a 4/2-2/1 schedule could minimize treatment-related toxicities; more importantly, patients with 4/2-2/1 schedule could achieve a superior survival benefit.
Keywords: clinical outcome, dosing schedule, metastatic renal cell carcinoma; safety profile; sunitinib.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Tolerability and outcome of sunitinib by giving 4/2 schedule versus 2/1 schedule in metastatic renal cell carcinoma patients: a prospective randomized multi-centric Egyptian study.Contemp Oncol (Pozn). 2020;24(4):221-228. doi: 10.5114/wo.2020.102802. Epub 2021 Jan 4. Contemp Oncol (Pozn). 2020. PMID: 33531869 Free PMC article.
-
Sunitinib administered on 2/1 schedule in patients with metastatic renal cell carcinoma: the RAINBOW analysis.Ann Oncol. 2015 Oct;26(10):2107-13. doi: 10.1093/annonc/mdv315. Epub 2015 Jul 27. Ann Oncol. 2015. PMID: 26216384
-
Superior tolerability of altered dosing schedule of sunitinib with 2-weeks-on and 1-week-off in patients with metastatic renal cell carcinoma--comparison to standard dosing schedule of 4-weeks-on and 2-weeks-off.Jpn J Clin Oncol. 2014 Mar;44(3):270-7. doi: 10.1093/jjco/hyt232. Epub 2014 Jan 27. Jpn J Clin Oncol. 2014. PMID: 24474815
-
Does an Alternative Sunitinib Dosing Schedule Really Improve Survival Outcomes over a Conventional Dosing Schedule in Patients with Metastatic Renal Cell Carcinoma? An Updated Systematic Review and Meta-Analysis.Cancers (Basel). 2019 Nov 21;11(12):1830. doi: 10.3390/cancers11121830. Cancers (Basel). 2019. PMID: 31766332 Free PMC article. Review.
-
A 2/1 Sunitinib Dosing Schedule Provides Superior Antitumor Effectiveness and Less Toxicity Than a 4/2 Schedule for Metastatic Renal Cell Carcinoma: A Systematic Review and Meta-Analysis.Front Oncol. 2020 Mar 6;10:313. doi: 10.3389/fonc.2020.00313. eCollection 2020. Front Oncol. 2020. PMID: 32211333 Free PMC article.
Cited by
-
Comparative Efficacy, Safety, and Costs of Sorafenib vs. Sunitinib as First-Line Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Meta-Analysis.Front Oncol. 2019 Jun 21;9:479. doi: 10.3389/fonc.2019.00479. eCollection 2019. Front Oncol. 2019. PMID: 31293962 Free PMC article.
-
Efficacy and Safety of Individualized Schedule of Sunitinib by Drug Monitoring in Patients with Metastatic Renal Cell Carcinoma.Cancer Manag Res. 2021 Aug 31;13:6833-6845. doi: 10.2147/CMAR.S327029. eCollection 2021. Cancer Manag Res. 2021. PMID: 34512023 Free PMC article.
-
Tolerability and outcome of sunitinib by giving 4/2 schedule versus 2/1 schedule in metastatic renal cell carcinoma patients: a prospective randomized multi-centric Egyptian study.Contemp Oncol (Pozn). 2020;24(4):221-228. doi: 10.5114/wo.2020.102802. Epub 2021 Jan 4. Contemp Oncol (Pozn). 2020. PMID: 33531869 Free PMC article.
-
Thyroid profile during the alternative Sunitinib dosing 2/1 schedule in metastatic renal cell carcinoma.Endocrine. 2020 Mar;67(3):597-604. doi: 10.1007/s12020-019-02088-4. Epub 2019 Nov 2. Endocrine. 2020. PMID: 31679139
-
Construction of a risk model and prediction of prognosis and immunotherapy based on cuproptosis-related LncRNAs in the urinary system pan-cancer.Eur J Med Res. 2023 Jun 27;28(1):198. doi: 10.1186/s40001-023-01173-9. Eur J Med Res. 2023. PMID: 37370148 Free PMC article.
References
-
- Janzen NK, Kim HL, Figlin RA. et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. - PubMed
-
- Faivre S, Delbaldo C, Vera K. et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. - PubMed
-
- Motzer RJ, Hutson TE, Tomczak P. et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. - PubMed
-
- Houk BE, Bello CL, Poland B. et al. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2010;66:357–371. - PubMed
LinkOut - more resources
Full Text Sources