Interleukin-6 is important for regulation of core body temperature during long-term cold exposure in mice
- PMID: 30271595
- PMCID: PMC6158403
- DOI: 10.3892/br.2018.1118
Interleukin-6 is important for regulation of core body temperature during long-term cold exposure in mice
Abstract
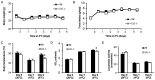
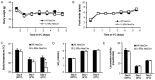
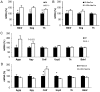
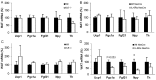
Interleukin-6 (IL6) is a cytokine important for inducing the fever response during infection and has been reported to uphold core body temperature during acute cold exposure. Recently it has also been indicated that IL6 in serum increases in cold-exposed mice. The aim of the present study was to investigate if IL6 is important for core body temperature regulation following a long-term cold exposure in mice. Experiments were performed with global IL6 deficient (-/-) mice, mice with conditional IL6 receptor α (IL6Rα) knockdown in the central nervous system (CNS; IL6RαNesCre) and appropriate wild-type (Wt) controls. All mice were placed in a cold environment (4°C) for 6 days. Core body temperature and oxygen consumption were measured by telemetry probes and indirect calorimetry at room temperature (20°C), and during the first and last day of cold exposure. Brain stem, hypothalamus and white and brown adipose tissues from the cold-exposed mice were subjected to gene expression analysis. After 6 days in 4°C, the IL6-/- mice exhibited significantly lower body temperature and oxygen consumption compared with Wt mice (P<0.05). The IL6RαNesCre mice also exhibited lower body temperature compared with WtNesCre controls during the last day of cold exposure (P<0.05). Furthermore, an increase in the mRNA level of brain-derived neurotrophic factor (Bdnf) was detected in the brain stem of both IL6-/- and IL6RαNesCre mice compared with the Wt groups (P<0.05). The finding that body temperature was decreased in IL6-/- and IL6RαNesCre mice indicates a decrease in thermogenesis in these animals. Bdnf has previously been indicated to increase body temperature and could in the present study be a mechanistic factor involved in counteracting the low body temperature in IL6-/- and IL6RαNesCre mice. These results suggest that IL6 is not only involved in body temperature regulation during infection, but also during long-term cold exposure, probably through mechanisms in the CNS.
Keywords: body temperature; brain stem; brown adipose tissue; cold exposure; energy expenditure; hypothalamus; interleukin-6; thermogenesis.
Figures
References
-
- Sundgren-Andersson AK, Ostlund P, Bartfai T. IL-6 is essential in TNF-alpha-induced fever. Am J Physiol. 1998;275:R2028–R2034. - PubMed
LinkOut - more resources
Full Text Sources
