Quantitation of paracetamol by liquid chromatography-mass spectrometry in human plasma in support of clinical trial
- PMID: 30271617
- PMCID: PMC6153454
- DOI: 10.4155/fsoa-2018-0039
Quantitation of paracetamol by liquid chromatography-mass spectrometry in human plasma in support of clinical trial
Abstract
Aim: Paracetamol is a well-tolerated antipyretic widely used in severe malaria management. The study aimed to develop and validate a rapid LC-MS/MS assay to quantify paracetamol in plasma from patients with severe malaria.
Materials & methods: Plasma sample was precipitated by organic solvent containing isotope-labeled paracetamol internal standard. Supernatant was isolated, diluted with water, followed by LC-MS/MS analysis.
Results: Plasma samples were extracted and assayed in less than 5.5 min. The assay response was linear (0.125-50 mg/l) with total intra- and interassay imprecision of <1.4%, which were considerably lower than most published reports.
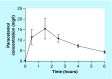
Conclusion: We developed, validated and applied a rapid and small volume LC-MS/MS assay with high precision and accuracy for plasma paracetamol quantitation in 989 samples from 62 patients with severe malaria. The simple and high-throughput quality could facilitate assay automation for future clinical studies.
Keywords: LC–MS/MS; acetaminophen; bioanalysis; malaria; paracetamol.
Conflict of interest statement
Financial & competing interests disclosure This study was part of Mahidol-Oxford Tropical Medicine Research Unit (MORU), Bangkok, Thailand core malaria research supported by the Wellcome Trust of Great Britain [grant number A-15–0001]. RK-T Kam, H-T Wong and the LC–MS/MS instruments were financially supported by Phase I Clinical Trial Center, The Chinese University of Hong Kong. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures


Similar articles
-
Simultaneous determination of paracetamol and chlorpheniramine in human plasma by liquid chromatography-tandem mass spectrometry.J Chromatogr A. 2000 Feb 18;870(1-2):77-86. doi: 10.1016/s0021-9673(99)01252-2. J Chromatogr A. 2000. PMID: 10722064
-
Agreement Between a Colorimetric Assay and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry for Quantifying Paracetamol Plasma Concentrations.AAPS J. 2024 Feb 1;26(1):23. doi: 10.1208/s12248-024-00890-1. AAPS J. 2024. PMID: 38302833
-
LC-MS/MS and GC-MS/MS measurement of plasma and urine di-paracetamol and 3-nitro-paracetamol: proof-of-concept studies on a novel human model of oxidative stress based on oral paracetamol administration.J Chromatogr B Analyt Technol Biomed Life Sci. 2014 May 15;959:71-81. doi: 10.1016/j.jchromb.2014.03.031. Epub 2014 Apr 2. J Chromatogr B Analyt Technol Biomed Life Sci. 2014. PMID: 24768921
-
Evaluation of a bracketing calibration-based isotope dilution liquid chromatography-tandem mass spectrometry candidate reference measurement procedure for 17α-hydroxyprogesterone in human plasma.Anal Bioanal Chem. 2019 Nov;411(27):7095-7104. doi: 10.1007/s00216-019-02086-5. Epub 2019 Oct 31. Anal Bioanal Chem. 2019. PMID: 31673753
-
Increasing liquid chromatography-tandem mass spectrometry throughput by mass tagging: a sample-multiplexed high-throughput assay for 25-hydroxyvitamin D2 and D3.Clin Chem. 2011 Mar;57(3):431-40. doi: 10.1373/clinchem.2010.157115. Epub 2011 Jan 18. Clin Chem. 2011. PMID: 21245371
Cited by
-
Leather Shaving Waste Extract as an Electrochemical Modifier at a Pencil Graphite Electrode for Paracetamol Determination in Pharmaceuticals.ACS Omega. 2025 Apr 29;10(18):18270-18282. doi: 10.1021/acsomega.4c08502. eCollection 2025 May 13. ACS Omega. 2025. PMID: 40385183 Free PMC article.
-
Simultaneous determination of tramadol and paracetamol in human plasma using LC-MS/MS and application in bioequivalence study of -fixed-dose combination.Ann Med. 2023;55(2):2270502. doi: 10.1080/07853890.2023.2270502. Epub 2023 Oct 19. Ann Med. 2023. PMID: 37857359 Free PMC article.
-
Efflux transporters in rat placenta and developing brain: transcriptomic and functional response to paracetamol.Sci Rep. 2021 Oct 6;11(1):19878. doi: 10.1038/s41598-021-99139-6. Sci Rep. 2021. PMID: 34615937 Free PMC article.
-
The Effect of Regularly Dosed Acetaminophen vs No Acetaminophen on Renal Function in Plasmodium knowlesi Malaria (PACKNOW): A Randomized, Controlled Trial.Clin Infect Dis. 2022 Oct 12;75(8):1379-1388. doi: 10.1093/cid/ciac152. Clin Infect Dis. 2022. PMID: 35180298 Free PMC article. Clinical Trial.
-
Intravenous Acetaminophen Does Not Provide Adequate Postoperative Analgesia in Dogs Following Ovariohysterectomy.Animals (Basel). 2021 Dec 20;11(12):3609. doi: 10.3390/ani11123609. Animals (Basel). 2021. PMID: 34944384 Free PMC article.
References
-
- World Health Organization. 20th Expert Committee, The selection and use of essential medicines. 2015. www.who.int/medicines/publications/essentialmedicines/EML2015_8-May-15.pdf
-
- Moore A, Collins S, Carroll D, McQuay H. Paracetamol with and without codeine in acute pain: a quantitative systematic review. Pain. 1997;70:193–201. - PubMed
-
- Bondarsky EE, Domingo AT, Matuza NM, Taylor MB, Thode HC, Singer AJ. Ibuprofen vs acetaminophen vs their combination in the relief of musculoskeletal pain in the ED: a randomized and controlled trial. Am. J. Emerg. Med. 2013;31:1357–1360. - PubMed
-
- Qi DS, May LG, Zimmerman B, et al. A randomized, double-blind, placebo-controlled study of acetaminophen 1000 mg versus acetaminophen 650 mg for the treatment of postsurgical dental pain. Clin. Ther. 2012;34:2247–2258. - PubMed
-
- Mitra S, Khandelwal P, Sehgal A. Diclofenac-tramadol vs diclofenac-acetaminophen combinations for pain relief after caesarean section. Acta Anaesthesiol. Scand. 2012;56:706–711. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
