Has the utilisation of Xpert® MTB/RIF in Manicaland Province, Zimbabwe, improved with new guidance on whom to test?
- PMID: 30271728
- PMCID: PMC6147069
- DOI: 10.5588/pha.18.0028
Has the utilisation of Xpert® MTB/RIF in Manicaland Province, Zimbabwe, improved with new guidance on whom to test?
Abstract
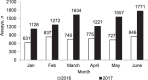
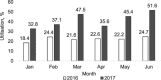
Setting: Manicaland Province, Zimbabwe. Objectives: To compare the utilisation and results of deploying Xpert® MTB/RIF in 13 (one provincial, six district and six rural) hospitals between January and June 2016, when Xpert was recommended only for those with presumptive multidrug-resistant tuberculosis (MDR-TB) and coinfection with human immunodeficiency virus (HIV), and between January and June 2017, when Xpert was recommended for all presumptive TB patients. Design: This was a cross-sectional study. Results: Xpert assays averaged 759 monthly in 2016 and 1430 monthly in 2017 (88% increase). Utilisation of Xpert averaged 22% monthly in 2016 and 42% in 2017 (88% increase). In 2017, utilisation of Xpert was significantly higher in provincial (82%) than in district (51%) and rural (26%) hospitals (P < 0.001). The proportion of successful assays that detected TB decreased significantly from 13% in 2016 to 7% in 2017 (a 46% decrease, P < 0.001); this phenomenon was observed in all types of hospital. The proportion of persons detected with rifampicin-resistant TB was similar between hospitals (4% in 2016 and 3% in 2017). The proportion of registered TB cases with bacteriological confirmation increased from 48% in 2016 to 53% in 2017 (P = 0.04). Conclusion: Xpert use in all presumptive TB patients led to a significant increase in assay numbers and utilisation of Xpert instruments, resulting in more bacteriological confirmation of cases.
Contexte : Province de Manicaland, Zimbabwe.Objectif : Comparer l'utilisation et les résultats du déploiement de l'Xpert® MTB/RIF dans 13 hôpitaux (1 provincial, 6 de district et 6 ruraux) entre janvier et juin 2016, quand l'Xpert a été recommandé seulement pour les patients ayant une présomption de la tuberculose (TB) multirésistante et une coinfection par le virus de l'immunodéficience humaine, et de janvier à juin 2017, quand l'Xpert a été recommandé pour tous les patients présumés TB.Schéma : Etude transversale.Résultats : Le nombre moyen de tests Xpert a été de 759 par mois en 2016 et de 1430 par mois en 2017 (augmentation de 88%). L'utilisation de l'instrument a été d'environ 22% par mois en 2016 et de 42% en 2017 (augmentation de 88%). En 2017, l'utilisation de l'instrument a été significativement plus élevée dans l'hôpital provincial (82%) comparé aux hôpitaux de district et ruraux (51% contre 26% ; P < 0,001). La proportion de succès des tests qui ont détecté une TB a significativement diminué de 13% en 2016 à 7% en 2017 (diminution de 46% ; P < 0,001) ; ceci a été observé dans tous les types d'hôpitaux. Les proportions de TB résistantes à la rifampicine ont été similaires entre les hôpitaux (4% en 2016 et 3% en 2017). La proportion des cas de TB enregistrés avec une confirmation bactériologique a augmenté de 48% en 2016 à 53% en 2017 (P = 0,04).Conclusion : L'utilisation du Xpert pour tous les patients présumés TB, les nombres de tests et l'utilisation de l'instrument Xpert ont significativement augmenté, aboutissant à davantage de confirmation bactériologique des cas.
Marco de referencia: La provincia de Manicaland, en Zimbabwe.Objetivos: Comparar la utilización de los dispositivos Xpert® MTB/RIF y los resultados de su despliegue en 13 hospitales (1 de provincia, 6 distritales y 6 rurales) durante dos períodos: de enero a junio del 2016, cuando se recomendaba la prueba Xpert solo en los casos de presunción de tuberculosis (TB) multirresistente y coinfección por el virus de la inmunodeficiencia humana, y de enero a junio del 2017, cuando se recomendaba la prueba Xpert en todos los pacientes con presunción de TB.Objetivos: Fue este un estudio transversal.Resultados: El promedio mensual de pruebas Xpert fue 759 en el 2016 y 1430 en el 2017 (un aumento del 88%). El porcentaje de utilización de los dispositivos Xpert fue en promedio 22% mensual en el 2016 y 42% en el 2017 (un aumento del 88%). En el 2017, la utilización de los dispositivos Xpert fue significativamente mayor en los hospitales de provincia (82%) comparados con los hospitales distritales y los rurales (51% contra 26%; P < 0,001). La proporción de análisis eficaces que detectaban la TB disminuyó de manera notable de 13% en el 2016 a 7% en el 2017 (una disminución del 46%; P < 0,001); esta situación se observó en todos los tipos de hospitales. La proporción de casos detectados de TB con resistencia a rifampicina fue equivalente en todos los hospitales (4% en el 2016 y 3% en el 2017). La proporción de casos de TB con confirmación bacteriológica registrados aumentó del 48% en el 2016 al 53% en el 2017 (P = 0,04).Conclusión: Al utilizar la prueba Xpert en todos los pacientes con presunción clínica de TB se aumentó considerablemente el número de pruebas y la utilización de los dispositivos Xpert, lo cual ha dado lugar a una mayor proporción de casos con confirmación bacteriológica.
Keywords: SORT IT; TB; Xpert® MTB/RIF; rifampicin-resistant TB.
Conflict of interest statement
Conflicts of interest: none declared.
Figures
References
-
- World Health Organization Global tuberculosis report, 2017. Geneva, Switzerland: WHO; 2017. WHO/HTM/TB/2017.23.
-
- Reid M J A, Shah N S. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis. 2009;9:173–184. - PubMed
-
- World Health Organization Policy statement: automated realtime time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.4. - PubMed
LinkOut - more resources
Full Text Sources
