IDLV-HIV-1 Env vaccination in non-human primates induces affinity maturation of antigen-specific memory B cells
- PMID: 30272013
- PMCID: PMC6125466
- DOI: 10.1038/s42003-018-0131-6
IDLV-HIV-1 Env vaccination in non-human primates induces affinity maturation of antigen-specific memory B cells
Abstract
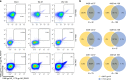
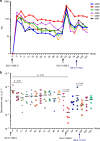
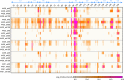
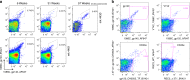
HIV continues to be a major global health issue. In spite of successful prevention interventions and treatment methods, the development of an HIV vaccine remains a major priority for the field and would be the optimal strategy to prevent new infections. We showed previously that a single immunization with a SIV-based integrase-defective lentiviral vector (IDLV) expressing the 1086.C HIV-1-envelope induced durable, high-magnitude immune responses in non-human primates (NHPs). In this study, we have further characterized the humoral responses by assessing antibody affinity maturation and antigen-specific memory B-cell persistence in two vaccinated macaques. These animals were also boosted with IDLV expressing the heterologous 1176.C HIV-1-Env to determine if neutralization breadth could be increased, followed by evaluation of the injection sites to assess IDLV persistence. IDLV-Env immunization was associated with persistence of the vector DNA for up to 6 months post immunization and affinity maturation of antigen-specific memory B cells.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

